In this issue of Blood, Masetti et al1 report on the findings of a multicenter observational study that explored the role of the microbiome in pediatric allogeneic hematopoietic stem cell transplantation (allo-HSCT). They studied 90 children and found that patients with high fecal microbial diversity prior to allo-HSCT had longer overall survival and a lower incidence of acute graft-versus-host disease (GVHD) compared to patients with low microbial diversity. In adult allo-HSCT recipients, high pretransplant fecal diversity has also been positively correlated with overall survival. Importantly, a key difference between this pediatric study and prior analyses in adults is that in this work, the perineutrophil-engraftment microbiome was not associated with outcome, whereas intestinal microbial features during this time window have been shown to predict outcome in adults (see figure).2,4
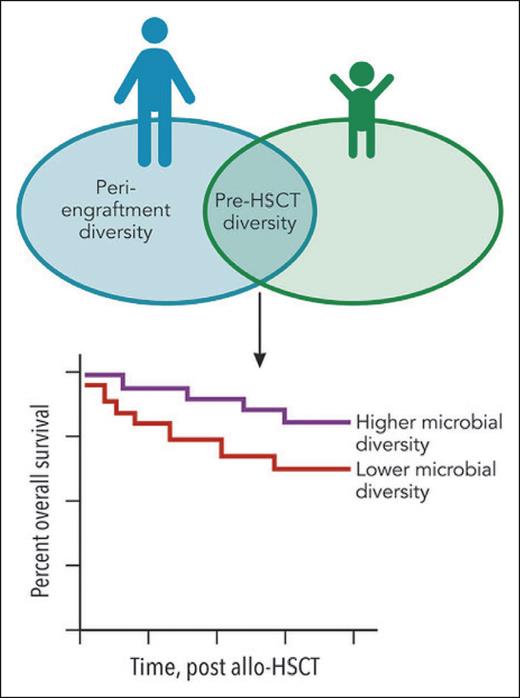
The Venn diagram shows the overlapping features in pediatric1 and adult2 allo-HSCT recipients. In adults, diversity of the pretransplant and peri-engraftment intestinal microbial communities has been associated with overall survival (OS): high diversity correlates with longer OS. In this pediatric study, the diversity of the pretransplant microbiome was correlated with OS, but the peri-engraftment time point was not. The survival curve denotes the common finding between pediatric1 and adult2,3 allo-HSCT recipients: ie, higher microbial diversity pretransplant is associated with improved OS. Professional illustration by Patrick Lane, ScEYEnce Studios.
The Venn diagram shows the overlapping features in pediatric1 and adult2 allo-HSCT recipients. In adults, diversity of the pretransplant and peri-engraftment intestinal microbial communities has been associated with overall survival (OS): high diversity correlates with longer OS. In this pediatric study, the diversity of the pretransplant microbiome was correlated with OS, but the peri-engraftment time point was not. The survival curve denotes the common finding between pediatric1 and adult2,3 allo-HSCT recipients: ie, higher microbial diversity pretransplant is associated with improved OS. Professional illustration by Patrick Lane, ScEYEnce Studios.
In adult HSCT recipients, outcomes linked with intestinal bacteria include overall survival,2,3 acute and chronic GVHD,5,6 infection,7 relapse,8 and immune reconstitution.4 Data regarding children undergoing allo-HSCT have thus far been lacking, and, therefore, the results from this study represent an important addition to the existing allo-HSCT literature.
Several factors may explain the differences between this pediatric study and previous work in the adult population. Firstly, the intestinal microbiome is likely to be more dynamic during childhood than in adulthood. Early studies suggested that the intestinal microbiome of children approximated the adult state early in life, at around the time children begin eating solid food.9 However, more recent data suggest that the microbiome of children continues to evolve and does not become comparable to that of adults until later in childhood. In addition, although Masetti et al's study is very important for the field and was rigorously and carefully analyzed, the cohort is smaller than many of the adult transplant cohorts in which the microbiome has been analyzed. A larger pediatric cohort, although undoubtedly challenging to recruit, may reveal further relationships and help to untangle confounders like dietary habits, antibiotic stewardship, or medication use that impact microbial diversity and community composition. Finally, the disease indications for allo-HSCT as well as allo-HSCT conditioning approaches in pediatric and adult populations differ. The incorporation of adult and pediatric patients in future studies may aid in clarifying the most important microbial contributors to transplant outcomes at any stage of life as well as precisely when their presence is required.
The specific mechanisms underpinning the relationship between intestinal bacteria and allo-HSCT outcomes remain unclear. One hypothesis is that a healthy, diverse intestinal microbiome is linked with favorable clinical outcomes owing to microbe-derived metabolites, such as the short-chain fatty acid butyrate. In this study, the “high diversity” stool samples were enriched for genera that are known short-chain fatty acid producers, including Blautia, Faecalibacterium, and Bacteroides. This finding aligns with the view that short-chain fatty acid production is immunomodulatory and promotes favorable outcomes in the transplant setting.10 This suggests that despite differences in the predictive time window between children and adults, there may be a common microbiome-associated “protective pathway” for allo-HSCT patients.
In addition, interventions aimed at mitigating or repairing microbial damage during the peri-transplant period are currently being studied in preclinical models and in active clinical trials. This important study from Masetti and colleagues suggests that these strategies are also likely to benefit children undergoing allo-HSCT.
Conflict-of-interest disclosure: M.S. reports an advisory role in A28 Therapeutics, an advisory board role in BMS, and consulting for Novartis. K.A.M. holds equity in and is on the advisory board of PostBiotics Plus and has consulted for Incyte.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal