In this issue of Blood, Graham et al1 and Karschnia et al2 provide new insights into the rare hypokinetic movement disorder (also termed movement and neurocognitive treatment emergent adverse events) that has been observed with BCMA-chimeric antigen receptor (CAR) T-cell treatment for multiple myeloma.
A subacute to late-emerging hypokinetic movement disorder with many features of parkinsonism was first reported in 5 patients enrolled in the CARTITUDE-1 study for ciltacabtagene autoleucel (cilta-cel).3-5 Patients presented weeks to months after CAR T-cell infusion with slowing of movements, limb rigidity, micrographia, tremor, and other typical parkinsonian symptoms. These symptoms were not fully reversible even with aggressive immunomodulation. However, many of the patients died from other causes, making the true reversibility uncertain. Subsequent clinical trials of the same product reported a lower but nonzero rate of parkinsonism. Puzzlingly, no analogous cases had been published until now for idecabtagene vicleucel (ide-cel), although there was mention of grade 3 parkinsonism in the package insert. For the first time, Karschnia et al now report a newly emergent movement disorder in a patient treated with ide-cel. More details on the timing and evolution of the symptoms will be helpful to ascertain whether this case truly falls within the spectrum of cases seen with cilta-cel.
Karschnia et al also examined risk factors for conventional immune effector cell-associated neurotoxicity syndrome (ICANS) in a mixed cohort of 76 patients treated with either cilta-cel or ide-cel in a real-world setting or with not otherwise specified BCMA-CAR T cells as part of a clinical trial. Of these patients, 40.8% developed ICANS, which was higher than the incidence reported in most of the pivotal studies for ide-cel and cilta-cel. Similar to what is seen with CD19-CAR T cells, cytokine release syndrome was identified as a major risk factor. Hypoalbuminemia was also associated with higher ICANS risk.
Two of the BCMA-CAR patients with ICANS later went on to develop parkinsonism, one on day 22 after ide-cel and the other on day 19 after cilta-cel. The latter patient is described in further detail by Graham et al, in a case that is notable for being the first report of fully reversible BCMA-CAR–associated movement disorder. There was a tight temporal correlation of emergence of neurologic symptoms and high counts of peripheral circulating CAR T cells. Symptoms completely resolved after CAR T cells were depleted with a single dose of cyclophosphamide. These findings suggest that there may be a causal relationship between the burden of circulating CAR T cells and parkinsonism symptoms. An on-target off-tumor activity against targets in the brain could be consistent with this picture, although the authors are careful to avoid any speculation that is not supported by their data. The fact that the symptoms were so readily reversible is encouraging, but also puzzling. If there is a destructive process at work, one would not expect such easy reversibility.
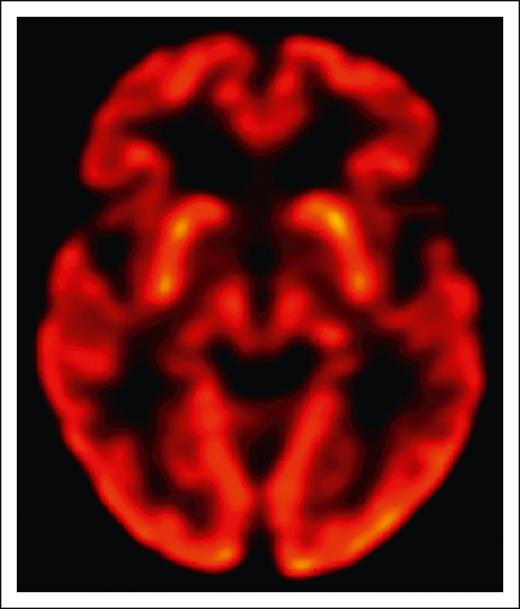
The overall gestalt of clinical examination findings in all patients reported to date is indeed most consistent with parkinsonism, a clinical syndrome that occurs with disruption of dopaminergic signaling in the brain. This can be due to loss of substantia nigra dopaminergic neurons in Parkinson disease, but also due to a variety of other mechanisms such as acquired striatal injury from stroke or hypoxia or drugs that interfere with dopaminergic neurotransmission. In the few BCMA-CAR patients where it has been reported, dopamine transporter imaging has been normal and histopathology has shown no loss of substantia nigra dopaminergic neurons. No patients with BCMA-CAR–associated parkinsonism have responded to carbidopa/levodopa. Instead, there is some evidence that the problem lies in the striatum, which receives dopaminergic innervation from the substantia nigra. Although the patient in Graham et al had normal positron emission tomography using fludeoxyglucose imaging (FDG-PET) of the basal ganglia (see figure), the ide-cel patients in Van Oekelen et al5 and in Karschnia et al had decreased uptake in the bilateral caudate nuclei. BCMA (=TNFRSF17) is detectable by RNA sequencing (RNA-seq) in the human striatum through development into young adulthood (www.brainspan.org). Since this finding comes from bulk RNA-seq data sets, it is unknown what cell type this constitutes. BCMA is not detectable by single-cell RNA-seq in other brain regions in adults (https://portal.brain-map.org/atlases-and-data/rnaseq/human-m1-10x). In adult striatum, there is no BCMA RNA detectable by in situ hybridization, and no protein is convincingly detectable by immunohistochemistry.6 Although this evidence is reassuring, we still cannot conclusively rule out low-level expression in normal adult brain.
The late onset of parkinsonism, multiple weeks to months after CAR T-cell infusion, may not be surprising. In other pathogenic processes, such as hypoxic injury or stroke to the basal ganglia, parkinsonism often emerges months to even years after the acute injury, presumably due to reorganization of neuronal circuits.7 Thus it is possible that an insult to the striatum may have occurred in the setting of CRS and ICANS, with the movement disorder not emerging until later, and potentially also spontaneously resolving over time. The single report of successful remission of parkinsonism after treatment with cyclophosphamide, although encouraging, should be treated with equipoise until further evidence becomes available.
Future investigations should continue to closely monitor patients for development of unusual toxicities. Timely expert clinical assessment is key, which can be an issue with patients receiving CAR T cells in a real-world setting. For BCMA-CAR patients with concern for movement disorder, this should include carefully documented serial neurologic examinations and advanced neuroimaging as directed by experts (such as FDG-PET and dopamine transporter imaging). As more sophisticated tools become available, we should reassess the presence of CAR targets in the brain. Efforts are underway to ensure more transparent case reporting, which will hopefully reduce confusion about the true incidence of these rare toxicities.
Normal uptake in the basal ganglia on brain FDG-PET in a BCMA-CAR T-cell patient with reversible parkinsonism. See Figure 1G in the article by Graham et al that begins on page 1248.
Normal uptake in the basal ganglia on brain FDG-PET in a BCMA-CAR T-cell patient with reversible parkinsonism. See Figure 1G in the article by Graham et al that begins on page 1248.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal