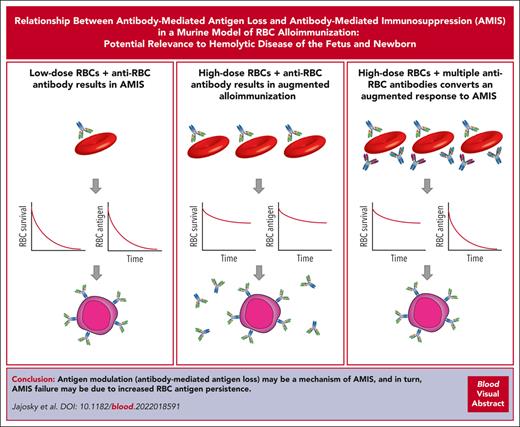
Key Points
CD4 T-cell proliferation can occur irrespective of whether antibodies induce AMIS.
Antibodies that rapidly remove the target antigen in the absence of RBC clearance can switch an augmented antibody response to AMIS.
Abstract
Antibodies against fetal red blood cell (RBC) antigens can cause hemolytic disease of the fetus and newborn (HDFN). Reductions in HDFN due to anti-RhD antibodies have been achieved through use of Rh immune globulin (RhIg), a polyclonal antibody preparation that causes antibody-mediated immunosuppression (AMIS), thereby preventing maternal immune responses against fetal RBCs. Despite the success of RhIg, it is only effective against 1 alloantigen. The lack of similar interventions that mitigate immune responses toward other RBC alloantigens reflects an incomplete understanding of AMIS mechanisms. AMIS has been previously attributed to rapid antibody-mediated RBC removal, resulting in B-cell ignorance of the RBC alloantigen. However, our data demonstrate that antibody-mediated RBC removal can enhance de novo alloimmunization. In contrast, inclusion of antibodies that possess the ability to rapidly remove the target antigen in the absence of detectable RBC clearance can convert an augmented antibody response to AMIS. These results suggest that the ability of antibodies to remove target antigens from the RBC surface can trigger AMIS in situations in which enhanced immunity may otherwise occur. In doing so, these results hold promise in identifying key antibody characteristics that can drive AMIS, thereby facilitating the design of AMIS approaches toward other RBC antigens to eliminate all forms of HDFN.
Introduction
Maternal development of alloantibodies toward red blood cell (RBC) alloantigens can destroy fetal RBCs and lead to fatal hemolytic disease of the fetus and newborn (HDFN).1-8 The only approach to actively prevent alloantibody formation is Rh immune globulin (RhIg) administration.9-15 RhIg is an anti-RhD polyclonal antibody preparation from individuals who are RhD alloimmunized, administered to prevent de novo anti-RhD antibody formation.16 Despite its success, RhIg immunoprophylaxis represents the only example of antibody-mediated immunosuppression (AMIS) used clinically and only targets a single RBC antigen.17,18 HDFN can arise from maternal immune responses against a variety of other RBC alloantigens.1-4 Indeed, alloimmunization toward non-ABO(H) and RhD alloantigens continues to occur in 0.1% to 1.1% of live births.19 As a result, additional forms of AMIS are needed if HDFN is to be prevented. Furthermore, although RhIg is clearly successful in preventing RhD alloimmunization, the reliance of this process on polyclonal RhIg will always be sensitive to risks associated with donor availability and infectious disease issues.20 However, because the mechanism by which AMIS occurs is incompletely understood, successful attempts to generate monoclonal antibody alternatives to RhIg or leverage AMIS to inhibit alloantibody responses against other RBC alloantigens have not been realized.
Antibody engagement of RBC alloantigens can have varying effects on de novo alloantibody development, depending on the target antigen and the antibody used.21-35 Attempts to generate monoclonal antibody alternatives to RhIg have produced mixed results, with some antibodies inducing AMIS, whereas others augment antibody formation.36,37 Varying outcomes after antibody binding, including differences in antibody-induced RBC clearance,38 can occur, suggesting that the ability of antibodies to affect RBC survival may ultimately influence target antigen availability for antibody induction and therefore the likelihood of AMIS.32,34,39 However, antibody-mediated RBC clearance does not always correlate with AMIS, suggesting that simple removal of RBCs may not be entirely responsible for the ability of some antibodies to prevent de novo antibody formation after RBC exposure. Indeed, recent studies demonstrate that AMIS may occur despite little to no RBC clearance.34,39 In these settings, antibody engagement appears to induce alterations to the target antigen, often referred to as antigen modulation, in which the target antigen is no longer detectable on the RBC surface despite RBC persistence in circulation.21,34,39,40 In this way, antigen loss is presumed to impact the ability of an ongoing immune response to lead to antibody formation after initial RBC alloantigen exposure.
Although antibodies can have differing effects on RBC clearance and AMIS, prior studies examining AMIS have also challenged recipients with varying RBC doses. Murine models, for example, have used RBC doses ranging from 107 RBCs (considered to approximate the amount of RBC exposure that may occur during a fetal-maternal hemorrhage) to 109 RBCs (roughly a 1-unit RBC exposure).33,39,41 Clinical studies examining AMIS have likewise varied with respect to RBC challenge dose, with different outcomes observed when distinct RBC doses were used.42,43 Despite these differences, no study has examined RBC challenge doses in parallel. Thus, it is not known whether the dose of RBCs used in AMIS studies, the types of antibodies used, or both, play a key role in defining the immunological outcome of RBC alloantigen exposure. Such considerations are important if the mechanisms that govern AMIS are to be understood and then leveraged to prevent maternal alloimmunization toward other RBC alloantigens that likewise cause HDFN.
The HOD alloimmunization model uses a target antigen consisting of a chimeric fusion protein containing hen egg lysozyme (HEL), a portion of ovalbumin (OVA), and Duffy expressed on murine RBCs. Because the HOD model is the most common system used to study antibody modification of RBC–induced antibody formation,32,39,42,44-49 we used this system to define the impact of RBC challenge dose and distinct antibody combinations on AMIS. The HOD model was designed such that HEL contains the B-cell epitopes, the partial sequence of OVA has CD4 T-cell (OT-II) epitopes, and Duffy acts to anchor the protein using its transmembrane domains.50 Our results demonstrate that RBC transfusion, in the presence of anti-Duffy antibodies, results in rapid RBC removal. However, the impact of RBC removal on de novo antibody formation depends on RBC challenge dose. Anti-Duffy antibodies induced AMIS after challenge with a low dose of HOD RBCs, whereas the opposite effect was observed with a high dose. In contrast, inclusion of anti-HEL antibodies, which independently induce rapid loss of the target antigen, with anti-Duffy antibodies, converted an otherwise augmented antibody response observed at the higher HOD RBC dose to AMIS. Taken together, these results demonstrate that antibody-induced antigen modulation can prevent antibody-induced enhancement of RBC alloimmunization, providing important insight into mechanisms of AMIS that may facilitate the rational design of additional approaches to prevent alloimmunization and therefore HDFN.
Materials and methods
Mice
C57BL/6 (B6) and OT-II mice were obtained from Charles River Laboratories and The Jackson Laboratory, respectively. These strains were crossbred to obtain Thy1.1+ OT-II mice. Fcer1g (common γ-chain Fc receptor [FcγR] knockout [KO]) mice were purchased from Taconic. HOD transgenic mice were maintained as previously described.50 Male and female mice aged 8 to 12 weeks were used. The experimental protocols and animal procedures performed in these studies were approved by the Institutional Animal Care and Use Committee.
RBC isolation, labeling, passive immunization, OT-II proliferation, and activation
B6 and HOD RBCs were collected and labeled as outlined previously.33,34,49,51-53 The 2F4 and 4B7 anti-HEL, MIMA29 anti-Duffy, and isotype control antibodies were injected as outlined previously.33,34,54 Pretreatment with 2.4G2 is detailed in the supplemental Methods, available on the Blood website. Mice were transfused and serum was collected as outlined previously.49,51,55 The direct antiglobulin test, antigen detection, C3 deposition, and Ter119 examination were performed as outlined previously.33,34,44,51,52,54,56,57 Carboxyfluorescein succinimidyl ester (CFSE)–labeled OT-II cells were evaluated by staining splenocytes with Brilliant Violet 785 anti-mouse CD3, BV650 anti-mouse CD4, APC anti-mouse Thy1.1, PECy5 anti-mouse CD69, and Alexa Fluor 700 anti-mouse CD44. Samples were run on a BD LSR II or Cytek Northern Lights flow cytometer and analyzed using FlowJo software as outlined previously.49,58
Creating HEL-linked RBCs
HEL (Worthington Biochemical Corporation), 1 mg in 0.1M 2-(N-morpholino) ethanesulfonic acid with 0.9% sodium chloride, pH 4.7 (MES buffer, ThermoFisher Scientific) was incubated with N-hydroxysulfosuccinimide (ThermoFisher Scientific) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and incubated for 15 minutes at room temperature. The N-hydroxysulfosuccinimide "activated" HEL was then desalted and buffer exchanged using Zeba spin desalting columns, 7K MWCO, 0.5 mL (ThermoFisher Scientific) in phosphate-buffered saline (PBS), pH 7.4. Immediately after purification, "activated" HEL was incubated with RBCs for 25 minutes at room temperature, followed by washing 3 times in PBS (pH 7.4) to remove unbound HEL.
Antibody detection, measurement of antibody affinity, glycan composition, and western blot analysis
To measure anti-HEL IgM and IgG antibodies, a flow-cytometry–based RBC crossmatch was performed. Packed HEL RBCs were incubated with serum, followed by antibody detection with anti-mouse IgM FITC or IgG APC (Jackson ImmunoResearch). The samples were then analyzed using the BD FACSCalibur flow cytometer.4,49,59-62 Antibody binding using surface plasmon resonance, solid phase microarray approaches, and flow cytometry were accomplished as outlined previously.63-65 Antibody glycan and western blot analysis were performed as previously described.40,55
Analysis of RBC uptake after transfusion
Splenocytes were stained with a Zombie Yellow Fixable Viability kit (Biolegend), followed by staining with anti-mouse CD11b PE-CF594 (clone M1/70) CD4 PE (clone GK1.5), CD169 PE (clone 3D6.112), CD3ε PerCP (clone 145-2C11), B220 PerCP (clone RA3-6B2), F4/80 PerCP (clone BM8), CD44 PE-Cy7 (clone IM7), I-Ab PE-Cy7 (clone AF6-120.1), Ly-6G APC-Cy7 (clone 1A8), F4/80 BV421 (clone BM8), CD8a BV421 (clone 53-6.7), TER-119 BV605, CD11c BV711 (clone N418), CD45R/B220 BV750 (clone RA3-6B2), CD3ε BV785 (clone 145-2C11), and CD86 BV785 (clone GL-1). All samples were analyzed on a BD LSR II or Cytek Northern Lights flow cytometer.45,58
Statistical analysis
Data were analyzed using FlowJo software. GraphPad Prism was used for statistical analysis. For comparisons between 2 groups, a Student t test was performed. One-way analysis of variance with Tukey multiple comparisons test was used to analyze ≥3 groups. A P value < .05 was defined as statistically significant.
See supplemental Methods for additional details.
Results
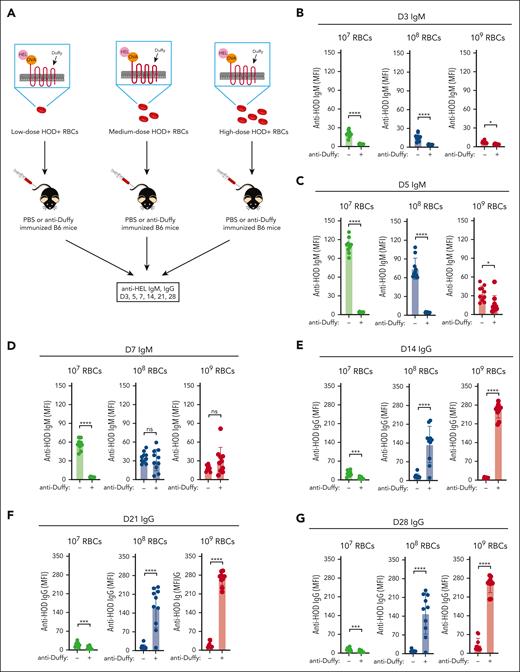
To define the role of RBC dose in AMIS, we transfused 107, 108, and 109 HOD RBCs to cover the breadth of doses used in prior studies,33,35,39,41,66 in the presence or absence of anti-Duffy antibodies (Figure 1A). Exposure to 107 HOD RBCs induced a significant anti-HEL IgM response (Figure 1B-D), with the corresponding anti-HEL IgG response being detectable but weaker (Figure 1E-G). Passive immunization with anti-Duffy antibodies blunted anti-HEL antibody formation, consistent with previous results,39 providing an example of AMIS. In contrast, exposure to 108 or 109 HOD RBCs in the presence of anti-Duffy resulted in the opposite outcome, with the anti-HEL IgG antibody response being >10-fold higher in the presence of anti-Duffy when compared with that in HOD RBC transfusion alone (Figure 1C).
The dose of transfused HOD RBCs and the presence of anti-Duffy antibodies influences RBC alloimmunization. (A) Schematic representation of nonimmunized or anti-Duffy–antibody passively immunized recipient B6 mice exposed to 107, 108, or 109 HOD RBCs followed by examination of anti-HOD antibody formation. (B-G) Nonimmunized or anti-Duffy–antibody passively immunized recipient B6 mice were exposed to 107, 108, or 109 HOD RBCs, followed by detection of anti-HEL IgM on days 3 (B), 5 (C), and 7 (D), and of IgG on days 14 (E), 21 (F), and 28 (G) after transfusion; n = 10 mice per group. All plots show mean values ± standard deviation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. These results are representative of 3 independent experiments.
The dose of transfused HOD RBCs and the presence of anti-Duffy antibodies influences RBC alloimmunization. (A) Schematic representation of nonimmunized or anti-Duffy–antibody passively immunized recipient B6 mice exposed to 107, 108, or 109 HOD RBCs followed by examination of anti-HOD antibody formation. (B-G) Nonimmunized or anti-Duffy–antibody passively immunized recipient B6 mice were exposed to 107, 108, or 109 HOD RBCs, followed by detection of anti-HEL IgM on days 3 (B), 5 (C), and 7 (D), and of IgG on days 14 (E), 21 (F), and 28 (G) after transfusion; n = 10 mice per group. All plots show mean values ± standard deviation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. These results are representative of 3 independent experiments.
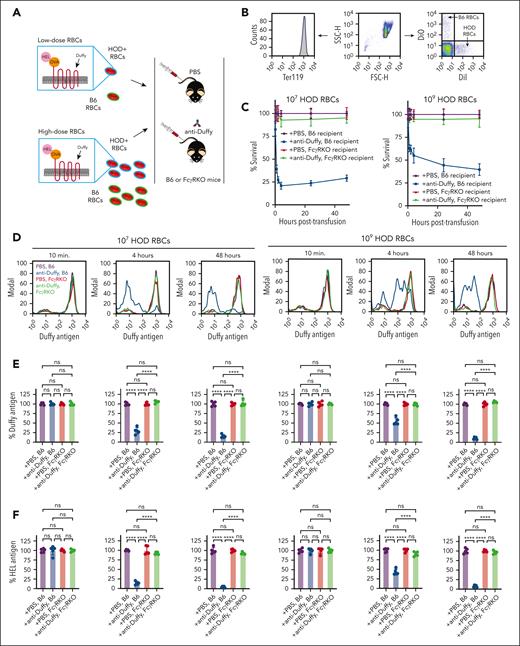
Given that HOD RBC dosage affected RBC alloimmunization, we defined the impact of anti-Duffy antibodies on HOD RBC clearance (Figure 2A). Because anti-Duffy antibodies enhance alloimmunization after exposure to 108 or 109 HOD RBCs, we simply compared 107 vs 109 HOD RBCs (the doses previously used in this same model system).39,42 HOD RBCs (107 or 109) experienced increased clearance in anti-Duffy passively immunized recipient B6 mice, with the lowest dose of HOD RBCs exhibiting the highest level of clearance (Figure 2B-C; supplemental Figure 1). Isotype controls failed to alter HOD RBC clearance or antibody induction (supplemental Figures 2 and 3), suggesting that these outcomes did not reflect nonspecific antibody-mediated modulation of the immune response. As anti-HOD antibodies may influence HOD RBC removal through activating FcγRs,32,54 we first examined whether recipient preexposure to the FcγR inhibitory antibody, 2.4G2,67 would prevent anti-Duffy–mediated antibody clearance. Although 2.4G2 certainly blunted HOD RBC clearance in anti-Duffy–immunized recipient mice, this inhibition was incomplete (supplemental Figures 4 and 5). As a result, we examined HOD RBC clearance in FcγR KO recipient mice. HOD RBCs did not demonstrate increased clearance in anti-Duffy–immunized FcγR KO recipient mice (Figure 2B-C), strongly suggesting that, regardless of the HOD RBC dose, anti-Duffy antibody-mediated removal depends on FcγRs.
Activating FcγRs affect anti-Duffy–induced HOD RBC clearance and antigen modulation. (A) Nonimmunized or anti-Duffy–antibody passively immunized B6 and FcγR KO mice were exposed to 107 or 109 DiI-labeled HOD RBCs followed by evaluation of HOD RBC survival and antigen levels. (B) Representative detection of DiI-labeled HOD RBCs and DiO-labeled B6 control RBCs in each recipient by flow cytometry. (C) Survival of 107 or 109 HOD RBCs in anti-Duffy–immunized or nonimmunized B6 or FcγR KO mice as indicated. (D) Representative histograms demonstrating Duffy antigen levels on DiI-labeled HOD RBCs at different time points in anti-Duffy–immunized or nonimmunized B6 or FcγR KO mice, as indicated. (E-G) Quantification of Duffy (E), HEL (F), and OVA (G) on HOD RBCs in anti-Duffy–immunized or nonimmunized B6 or FcγR KO mice, as indicated. Modal = all histograms scaled as a percentage of the maximum count; n = 5 mice per group. All plots show mean values ± standard deviation. ∗P < .05; ∗∗∗P < .001; and ∗∗∗∗P < .0001. These results are representative of 3 independent experiments.
Activating FcγRs affect anti-Duffy–induced HOD RBC clearance and antigen modulation. (A) Nonimmunized or anti-Duffy–antibody passively immunized B6 and FcγR KO mice were exposed to 107 or 109 DiI-labeled HOD RBCs followed by evaluation of HOD RBC survival and antigen levels. (B) Representative detection of DiI-labeled HOD RBCs and DiO-labeled B6 control RBCs in each recipient by flow cytometry. (C) Survival of 107 or 109 HOD RBCs in anti-Duffy–immunized or nonimmunized B6 or FcγR KO mice as indicated. (D) Representative histograms demonstrating Duffy antigen levels on DiI-labeled HOD RBCs at different time points in anti-Duffy–immunized or nonimmunized B6 or FcγR KO mice, as indicated. (E-G) Quantification of Duffy (E), HEL (F), and OVA (G) on HOD RBCs in anti-Duffy–immunized or nonimmunized B6 or FcγR KO mice, as indicated. Modal = all histograms scaled as a percentage of the maximum count; n = 5 mice per group. All plots show mean values ± standard deviation. ∗P < .05; ∗∗∗P < .001; and ∗∗∗∗P < .0001. These results are representative of 3 independent experiments.
Previous studies suggest that in addition to inducing RBC clearance, anti-Duffy antibodies can induce antigen modulation, a process whereby antigen undergoes reduced detectability on RBC surface. To study antigen modulation, we exposed B6 or FcγR KO mice to 107 or 109 HOD RBCs in the presence or absence of anti-Duffy antibodies, and measured antibody binding and HOD antigen levels. Anti-Duffy antibody binding could be readily detected on HOD RBCs only in anti-Duffy antibody–immunized recipient mice (supplemental Figure 6). By 4 hours after transfusion, the level of HEL, OVA, and Duffy antigens were significantly lower in mice passively immunized with anti-Duffy antibodies (Figure 2D-G). Similar to the inability of 2.4G2 to prevent anti-Duffy–mediated HOD RBC clearance, 2.4G2 failed to significantly impact anti-Duffy–mediated antigen loss (supplemental Figures 4 and 5). In contrast, although anti-Duffy antibodies were detected on the surface of HOD RBCs in immunized FcγR KO mice, less antigen loss was observed in FcγR KO mice (Figure 2D-G). In contrast, TER119 levels remained unchanged (supplemental Figure 6). Consistent with the role of FcγRs in anti-Duffy antibody–mediated HOD RBC clearance, little to no complement component 3 (C3) was detected on HOD RBCs (supplemental Figure 6).
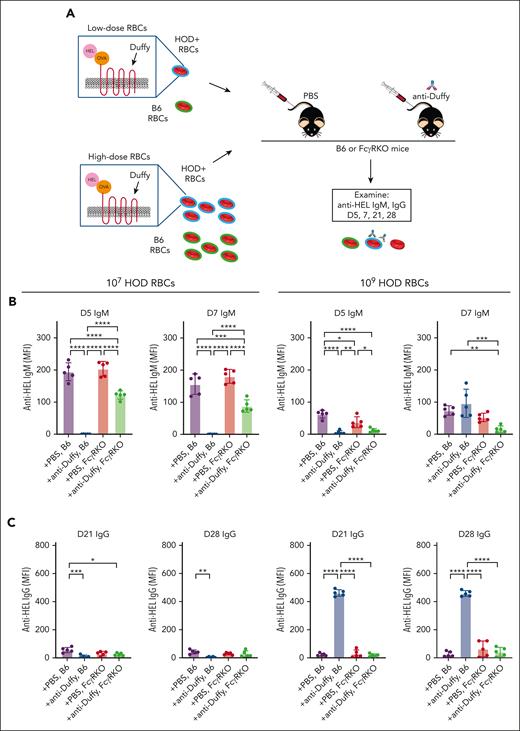
Given the role of FcγRs in both RBC clearance and antigen modulation, we sought to determine whether FcγRs mediate suppression or augmentation of anti-HOD antibody formation. To accomplish this, B6 and FcγR KO mice were exposed to 107 or 109 HOD RBCs in the presence or absence of anti-Duffy antibodies (Figure 3A). Compared with recipient B6 mice, low-dose HOD RBC exposure in the presence of anti-Duffy antibodies in recipient FcγR KO mice failed to experience similar reductions in anti-HEL IgM or IgG antibody formation (Figure 3B-C). High-dose HOD RBC exposure in the presence of anti-Duffy antibodies in FcγR KO likewise failed to result in the same level of increased anti-HEL IgG antibody formation (Figure 3C). Similar to the inability of 2.4G2 to prevent anti-Duffy–mediated clearance or antigen modulation, treatment with 2.4G2 failed to completely reverse the influence of anti-Duffy modulation on HOD RBC alloimmunization (supplemental Figure 7). These results suggest that FcγRs are required for anti-Duffy antibody-induced suppression or augmentation of anti-HEL antibody formation.
The presence of activating FcγRs affects antibody-induced alterations in RBC alloimmunization. (A) Schematic of the experimental design. Anti-Duffy–antibody immunized or nonimmunized B6 or FcγR KO mice were exposed to different doses (107 or 109) of HOD followed by evaluation of anti-HOD antibody formation. (B-C) Anti-HEL IgM (B) and IgG (C) antibody responses were measured in anti-Duffy–immunized or nonimmunized B6 or FcγR KO mice after exposure to 107 and 109 HOD RBCs at various time points, as indicated; n = 5 mice per group. All plots show mean values ± standard deviation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. These results are representative of 2 independent experiments.
The presence of activating FcγRs affects antibody-induced alterations in RBC alloimmunization. (A) Schematic of the experimental design. Anti-Duffy–antibody immunized or nonimmunized B6 or FcγR KO mice were exposed to different doses (107 or 109) of HOD followed by evaluation of anti-HOD antibody formation. (B-C) Anti-HEL IgM (B) and IgG (C) antibody responses were measured in anti-Duffy–immunized or nonimmunized B6 or FcγR KO mice after exposure to 107 and 109 HOD RBCs at various time points, as indicated; n = 5 mice per group. All plots show mean values ± standard deviation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. These results are representative of 2 independent experiments.
Next, we sought to determine whether anti-Duffy–induced HOD RBC clearance may result in the differential uptake of HOD RBCs in a dose-sensitive manner. To accomplish this, B6 or FcγR KO mice were exposed to 107 or 109 CFSE-labeled HOD RBCs in the presence or absence of anti-Duffy antibodies (Figure 4A). Using this approach, we were unable to consistently detect HOD RBCs when only 107 cells were transfused, regardless of the recipient or the presence or absence of anti-Duffy antibodies. However, CFSE+ cells could readily be detected in recipients that received 109 HOD RBCs (Figure 4C-E). Enhanced HOD RBC uptake was particularly apparent in neutrophils, red pulp macrophages, and dendritic cells (DCs) (Figure 4C-E). In addition, CD86, an activation marker for red pulp macrophages and DCs, increased significantly in B6 mice passively immunized with anti-Duffy antibodies. Changes in HOD RBC uptake appeared to be dictated by FcγRs, because similar uptake of HOD RBCs was not observed in FcγR KO mice despite the presence of anti-Duffy antibodies. CFSE positivity in each immune population evaluated appeared to largely reflect phagocytosis as opposed to tethering of HOD RBCs at the cell surface, because the majority of CFSE+ immune populations detected were negative for the RBC surface marker TER119 (Figure 4C-E).
Activating FcγRs regulate anti-Duffy–mediated HOD RBC removal by multiple immune populations in the spleen. (A) Schematic of experiment shown. CFSE-labeled HOD RBCs were transfused into B6 or FcγR KO mice in the presence or absence of anti-Duffy antibodies. (B) A partial summary of the flow-cytometry gating strategy used to detect CFSE+ immune populations. (C-E) Examination of distinct immune populations for CFSE positivity after exposure of B6 or FcγR KO mice to CFSE-labeled HOD RBCs in the presence or absence of anti-Duffy antibodies, including neutrophils (PMNs) (C), red pulp macrophages (RPMs; D), and DCs (E); n = 3 to 4 mice per group. Quantitative analysis of CFSE+ TER119− events are shown. All plots show mean values ± standard deviation. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001. These results are representative of 3 independent experiments.
Activating FcγRs regulate anti-Duffy–mediated HOD RBC removal by multiple immune populations in the spleen. (A) Schematic of experiment shown. CFSE-labeled HOD RBCs were transfused into B6 or FcγR KO mice in the presence or absence of anti-Duffy antibodies. (B) A partial summary of the flow-cytometry gating strategy used to detect CFSE+ immune populations. (C-E) Examination of distinct immune populations for CFSE positivity after exposure of B6 or FcγR KO mice to CFSE-labeled HOD RBCs in the presence or absence of anti-Duffy antibodies, including neutrophils (PMNs) (C), red pulp macrophages (RPMs; D), and DCs (E); n = 3 to 4 mice per group. Quantitative analysis of CFSE+ TER119− events are shown. All plots show mean values ± standard deviation. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001. These results are representative of 3 independent experiments.
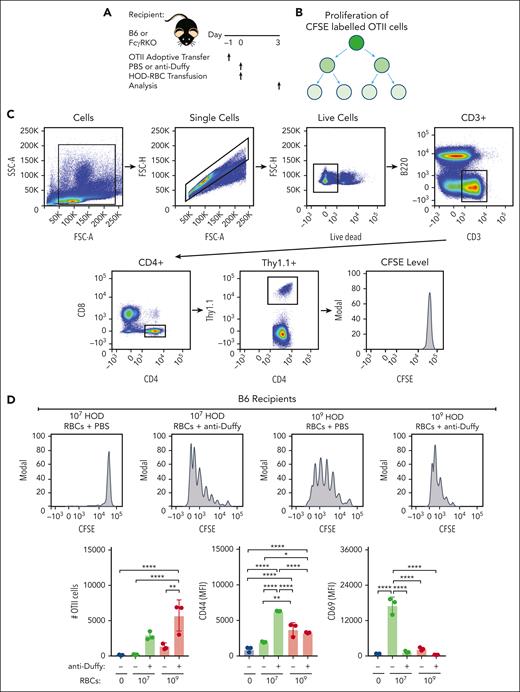
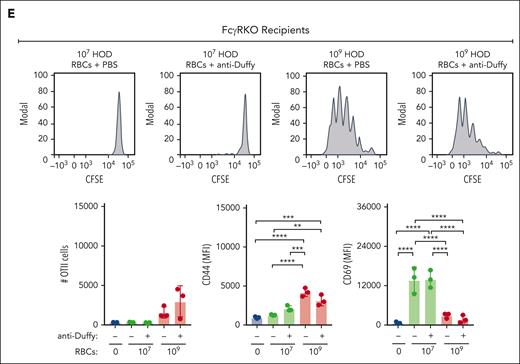
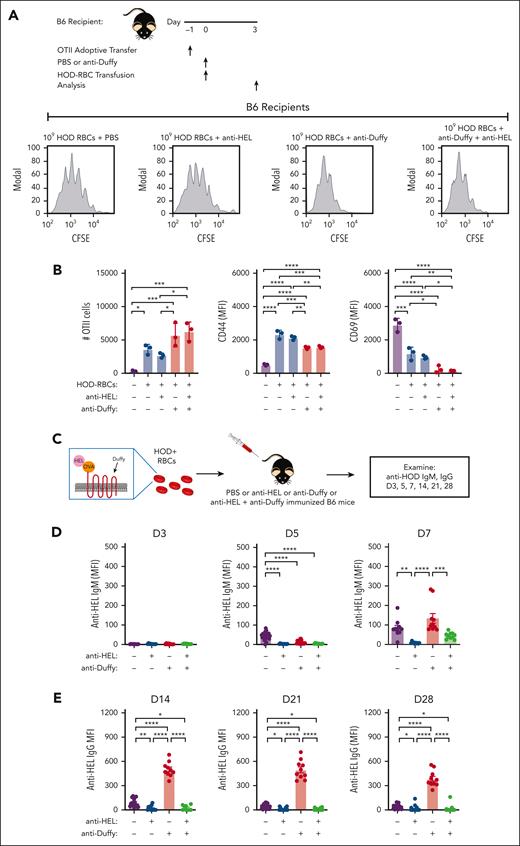
Because DCs are required for the development of anti-HOD antibodies and because anti-Duffy antibodies increased DC uptake,45,46 anti-Duffy antibody-mediated enhancement of the HOD immune response may in part reflect increased HOD RBC uptake and subsequent CD4 T-cell proliferation. To test this, CFSE-labeled OT-II CD4 T transgenic cells were adoptively transferred to B6 or FcγR KO mice to facilitate detection of CD4 T-cell proliferation after exposure to 107 or 109 HOD RBCs (Figure 5A-C). Using this approach, we found very limited CD4 T-cell proliferation after exposure to 107 HOD RBCs (Figure 5D), consistent with the reduced anti-HEL IgG response observed at this dose. In contrast, robust CD4 T-cell proliferation could be detected after exposure to 109 HOD RBCs. Despite inhibiting anti-HEL antibody formation, inclusion of anti-Duffy antibodies enhanced CD4 T-cell proliferation after exposure to 107 HOD RBCs (Figure 5D). Anti-Duffy antibodies also increased CD4 T-cell proliferation after exposure to 109 HOD RBCs, whereas no anti-Duffy antibody-mediated increase in CD4 T-cell proliferation was observed after exposure to low and high-dose HOD RBCs in FcγR KO mice (Figure 5E; supplemental Figure 8). In addition, differences in CD44 and CD69 activation markers mediated by anti-Duffy antibodies after exposure to either low- or high-dose HOD RBCs in recipient B6 mice did not occur in recipient FcγR KO mice (Figure 5E). These results demonstrate that anti-Duffy antibodies enhance CD4 T-cell proliferation after exposure to low-dose HOD RBCs, despite inhibiting, or even preventing, de novo antibody formation.
OVA323-339–specific CD4 T-cell (OT-II) proliferation depends on the dose of HOD RBCs and presence of anti-Duffy antibodies. (A) Schematic of the experimental design used to analyze CD4+ OT-II proliferation and activation after exposure to HOD RBCs in the presence or absence of anti-Duffy antibodies. (B) Schematic showing OT-II proliferation by CFSE dilution with each round of division. (C) Gating strategy used to examine the proliferation of CFSE–labeled OT-II cells after exposure of B6 or FcγR KO mice to 107 or 109 HOD RBCs in the presence or absence of anti-Duffy antibodies. (D) Representative flow-cytometry data generated after exposure of B6 mice to 107 or 109 HOD RBCs in the presence or absence of anti-Duffy antibodies. Quantitative analysis of OT-II proliferation, CD44, and CD69 levels after exposure of B6 mice to 107 or 109 HOD RBCs in the presence or absence of anti-Duffy antibodies. (E) Representative flow-cytometry data generated after exposure of FcγR KO mice to 107 or 109 HOD RBCs in the presence or absence of anti-Duffy antibodies. Quantitative analysis of OT-II proliferation, CD44, and CD69 levels after exposure of FcγR KO mice to 107 or 109 HOD RBCs in the presence or absence of anti-Duffy antibodies. n = 3 mice per group. Modal = all histograms scaled as a percentage of the maximum count. All plots show mean values ± standard deviation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. These results are representative of 3 independent experiments.
OVA323-339–specific CD4 T-cell (OT-II) proliferation depends on the dose of HOD RBCs and presence of anti-Duffy antibodies. (A) Schematic of the experimental design used to analyze CD4+ OT-II proliferation and activation after exposure to HOD RBCs in the presence or absence of anti-Duffy antibodies. (B) Schematic showing OT-II proliferation by CFSE dilution with each round of division. (C) Gating strategy used to examine the proliferation of CFSE–labeled OT-II cells after exposure of B6 or FcγR KO mice to 107 or 109 HOD RBCs in the presence or absence of anti-Duffy antibodies. (D) Representative flow-cytometry data generated after exposure of B6 mice to 107 or 109 HOD RBCs in the presence or absence of anti-Duffy antibodies. Quantitative analysis of OT-II proliferation, CD44, and CD69 levels after exposure of B6 mice to 107 or 109 HOD RBCs in the presence or absence of anti-Duffy antibodies. (E) Representative flow-cytometry data generated after exposure of FcγR KO mice to 107 or 109 HOD RBCs in the presence or absence of anti-Duffy antibodies. Quantitative analysis of OT-II proliferation, CD44, and CD69 levels after exposure of FcγR KO mice to 107 or 109 HOD RBCs in the presence or absence of anti-Duffy antibodies. n = 3 mice per group. Modal = all histograms scaled as a percentage of the maximum count. All plots show mean values ± standard deviation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. These results are representative of 3 independent experiments.
Given the lack of change in HOD RBC clearance or antigen modulation in immunized FcγR KO mice, coupled with the reduced ability of anti-Duffy antibodies to influence anti-HEL antibody outcomes in FcγR KO mice, antibody-mediated HOD RBC clearance and antigen modulation may ultimately converge to dictate de novo alloantibody formation when anti-Duffy antibodies are present. One possibility is that a threshold of antigen must be present to sufficiently engage the immune system after initial HOD RBC clearance and CD4 T-cell proliferation to provide sufficient antigen substrate for a sustained immune response. To initially examine this, we simply compared the relative level of antigen availability, which is dictated by HOD RBC clearance and antigen modulation, after 107 or 109 HOD RBC exposure. Although HOD RBCs experienced rapid clearance after exposure to anti-Duffy–immunized mice at both doses, the total level of HEL antigen remaining on circulating HOD RBCs differed considerably, with exposure to 109 HOD RBCs resulting in persistent levels of HEL antigen (20% remaining at 4 hours after transfusion) that far exceeded those observed after 107 HOD RBC exposure (3.2% remaining at 4 hours after transfusion) (Figure 2). These results raise the possibility that, despite the ability of anti-Duffy antibodies to enhance CD4 T-cell proliferation irrespective of the HOD RBC exposure dose, differences in the persistence of HEL antigen levels may account for the differential outcome observed after exposure to distinct HOD RBC doses.
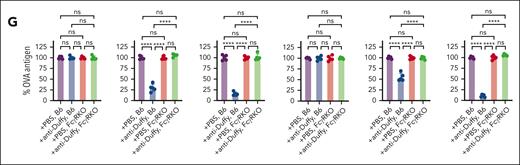
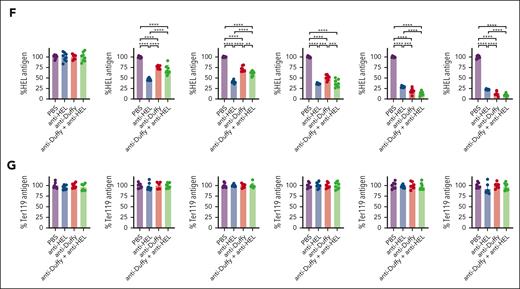
To directly examine whether persisting antigen on HOD RBCs may facilitate anti-Duffy antibody-mediated enhancement of HOD RBC alloimmunization at higher HOD RBC doses, we next included 2 additional antibodies that directly target the HEL antigen (Figure 6A). Anti-HEL antibodies reportedly rapidly reduce HEL antigen levels independent of FcγRs or complement.34 Because exposure to 107 HOD RBCs in the presence of anti-Duffy antibodies reduced anti-HEL antibody formation, we focused this study on the impact of anti-HEL with anti-Duffy antibodies on the immune response to 109 HOD RBCs. We first determined whether anti-HEL antibodies possess the ability to induce rapid loss of HEL even in the presence of anti-Duffy antibodies. This was accomplished by transfusing nonimmunized or passively immunized recipient mice with anti-Duffy, anti-HEL, or both, followed by examination of HOD RBC clearance and levels of the HEL, OVA, Duffy, and TER-119 (Figure 6). Recipients immunized with anti-Duffy antibodies cleared HOD RBCs regardless of whether anti-HEL antibodies were likewise present, whereas anti-HEL antibodies failed to alter RBC survival (Figure 6B; supplemental Figure 9). In contrast to HEL antigen levels observed after exposure to anti-Duffy antibodies alone, anti-HEL antibodies alone or in combination with anti-Duffy antibodies induced a rapid decline in HEL antigen levels, 1 hour after transfusion (Figure 6F). This strongly suggests that anti-HEL antibodies can remove the HEL antigen irrespective of anti-Duffy–induced HOD RBC clearance or antigen removal. In contrast, OVA and Duffy levels were largely unaffected by anti-HEL antibodies (Figure 6C-E), whereas inclusion of isotype controls failed to alter HOD RBC clearance or antigen levels (supplemental Figure 10). Importantly, TER119 antigen levels were unaffected by inclusion of antibodies regardless of the combination (Figure 6G). Anti-Duffy and anti-HEL antibodies exhibited distinct affinities for their target epitopes and likewise possessed distinct glycosylation patterns (supplemental Figures 11 and 12), which, along with the unique targets they engage, may, in part, account for differences in outcomes observed after HOD RBC engagement. Antibody engagement with either anti-Duffy, anti-HEL, or anti-HEL and anti-Duffy resulted in loss of the target antigen, which could not be detected in plasma (supplemental Figure 13).
Anti-HEL antibodies accelerate loss of HEL antigen loss in the presence or absence of anti-Duffy without affecting RBC removal. (A) Schematic of the experimental design. Anti-HEL–, anti-Duffy–, and anti-HEL + anti-Duffy–immunized B6 mice were exposed to high-dose (109) DiI-labeled HOD RBCs, followed by evaluation of HOD RBC survival and evaluation of HEL, OVA, Duffy, and Ter119 cell surface levels. (B) Quantitative analysis of HOD RBC survival in nonimmunized or anti-HEL–, anti-Duffy–, and anti-HEL + anti-Duffy–immunized B6 mice. (C) Representative flow-cytometry histograms demonstrating the level of OVA antigen on HOD RBCs after exposure of nonimmunized or anti-HEL–, anti-Duffy–, and anti-HEL + anti-Duffy–immunized B6 mice to HOD RBCs. (D-G) Quantitative analysis of the levels of OVA (D), Duffy (E), HEL (F), and TER119 (G) on HOD RBCs in nonimmunized or anti-HEL–, anti-Duffy–, and anti-HEL + anti-Duffy–immunized B6 mice at the indicated times. n = 5 mice per group. Modal = all histograms scaled as a percentage of the maximum count. All plots show mean values ± standard deviation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. These results are representative of 2 independent experiments.
Anti-HEL antibodies accelerate loss of HEL antigen loss in the presence or absence of anti-Duffy without affecting RBC removal. (A) Schematic of the experimental design. Anti-HEL–, anti-Duffy–, and anti-HEL + anti-Duffy–immunized B6 mice were exposed to high-dose (109) DiI-labeled HOD RBCs, followed by evaluation of HOD RBC survival and evaluation of HEL, OVA, Duffy, and Ter119 cell surface levels. (B) Quantitative analysis of HOD RBC survival in nonimmunized or anti-HEL–, anti-Duffy–, and anti-HEL + anti-Duffy–immunized B6 mice. (C) Representative flow-cytometry histograms demonstrating the level of OVA antigen on HOD RBCs after exposure of nonimmunized or anti-HEL–, anti-Duffy–, and anti-HEL + anti-Duffy–immunized B6 mice to HOD RBCs. (D-G) Quantitative analysis of the levels of OVA (D), Duffy (E), HEL (F), and TER119 (G) on HOD RBCs in nonimmunized or anti-HEL–, anti-Duffy–, and anti-HEL + anti-Duffy–immunized B6 mice at the indicated times. n = 5 mice per group. Modal = all histograms scaled as a percentage of the maximum count. All plots show mean values ± standard deviation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. These results are representative of 2 independent experiments.
The HEL antigen is the primary target of the humoral immune response to HOD, whereas OVA is thought to support the T-cell response. To determine whether anti-HEL antibodies with or without anti-Duffy antibodies affect the CD4 T-cell response, OT-II proliferation was measured after exposure to HOD RBCs alone, or HOD RBCs in the presence of anti-Duffy, anti-HEL, or both (Figure 7A; supplemental Figure 14). Consistent with the retention of OVA despite loss of the HEL antigen in anti-HEL–treated mice, CD4 T-cell proliferation was unaffected by anti-HEL antibodies (Figure 7B; supplemental Figure 14). Similarly, and perhaps more importantly, the enhancement of CD4 T-cell proliferation observed after HOD RBC exposure in anti-Duffy antibody–immunized mice was not affected by the presence of anti-HEL antibodies (Figure 7B; supplemental Figure 14). To determine whether anti-HEL antibodies can affect alloimmunization in the presence of anti-Duffy antibodies, HOD RBCs were transfused into mice immunized with either anti-HEL, anti-Duffy, or anti-HEL with anti-Duffy. Anti-HEL antibodies alone reduced anti-HEL IgM and IgG at day 7 and day 28, respectively, compared with PBS-treated mice (Figure 7C-E). In contrast, isotype controls failed to alter HOD RBC–induced antibody formation (supplemental Figure 15). In comparison with anti-Duffy immunization alone, anti-HEL with anti-Duffy antibodies reduced anti-HEL IgM and IgG alloimmunization. These results demonstrate that anti-HEL antibodies can inhibit the overall anti-HOD antibody response, despite the ability of anti-Duffy antibodies to enhance CD4 T-cell proliferation in the presence or absence of anti-HEL antibodies after HOD RBC exposure (supplemental Table 1).
Anti-HEL antibodies reverse the ability of anti-Duffy to augment HOD RBC alloimmunization. (A) The experimental design of CD4+ OT-II cell evaluation after exposure of B6 mice to HOD RBCs in the presence or absence of anti-HEL, anti-Duffy or anti-HEL + anti-Duffy antibodies, including representative flow cytometric examination of OT-II proliferation after exposure to 109 HOD RBCs in the presence or absence of anti-HEL, anti-Duffy or anti-HEL + anti-Duffy antibodies, as indicated. (B) Quantitative analysis of OT-II proliferation, CD44, and CD69 levels after exposure of B6 mice to high-dose (109) HOD RBCs in the presence or absence of anti-HEL, anti-Duffy or anti-HEL + anti-Duffy antibodies, as indicated. (C) Schematic of HOD RBC–induced antibody formation in the presence or absence of anti-HEL, anti-Duffy, or anti-HEL + anti-Duffy antibodies. (D-E) Evaluation of the development of the anti-HEL IgM (D) and IgG (E) antibody response in the presence or absence of anti-HEL, anti-Duffy, or anti-HEL + anti-Duffy antibodies after exposure of B6 mice to 109 HOD RBCs at the indicated time points. n = 3 to 10 mice per group. Modal = all histograms scaled as a percentage of the maximum count. All plots show mean values ± standard deviation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. These results are representative of 2 independent experiments.
Anti-HEL antibodies reverse the ability of anti-Duffy to augment HOD RBC alloimmunization. (A) The experimental design of CD4+ OT-II cell evaluation after exposure of B6 mice to HOD RBCs in the presence or absence of anti-HEL, anti-Duffy or anti-HEL + anti-Duffy antibodies, including representative flow cytometric examination of OT-II proliferation after exposure to 109 HOD RBCs in the presence or absence of anti-HEL, anti-Duffy or anti-HEL + anti-Duffy antibodies, as indicated. (B) Quantitative analysis of OT-II proliferation, CD44, and CD69 levels after exposure of B6 mice to high-dose (109) HOD RBCs in the presence or absence of anti-HEL, anti-Duffy or anti-HEL + anti-Duffy antibodies, as indicated. (C) Schematic of HOD RBC–induced antibody formation in the presence or absence of anti-HEL, anti-Duffy, or anti-HEL + anti-Duffy antibodies. (D-E) Evaluation of the development of the anti-HEL IgM (D) and IgG (E) antibody response in the presence or absence of anti-HEL, anti-Duffy, or anti-HEL + anti-Duffy antibodies after exposure of B6 mice to 109 HOD RBCs at the indicated time points. n = 3 to 10 mice per group. Modal = all histograms scaled as a percentage of the maximum count. All plots show mean values ± standard deviation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. These results are representative of 2 independent experiments.
Discussion
The results of this study suggest that enhanced antigen modulation in the face of antibody-induced RBC clearance can ultimately result in AMIS. These findings also demonstrate that AMIS can be dissociated from CD4 T-cell proliferation and that the ability of antibodies to induce RBC clearance and rapid antigen removal may be a critical in driving AMIS. These findings contribute to growing evidence that antibody engagement of antigen does not uniformly result in RBC clearance but that antigen modulation can likewise occur.40,68-70 Given the ability of alloimmunization to cause HDFN after exposure to a variety of antigens, these findings may provide insight into governing principles whereby AMIS may occur. In doing so, these results may directly facilitate the identification of monoclonal antibody alternatives to polyclonal RhIg or alternative AMIS formularies against non-RhD antigens.
Prior studies have elegantly demonstrated that combinations of antibodies could further enhance the ability of individual antibodies to induce AMIS, whereas enhanced CD4 T-cell proliferation can accompany antibody-induced enhancement of de novo antibody development.32,35 A variety of antibody features, including antibody glycosylation, can affect these outcomes.43 However, whether AMIS reflects general immune ignorance, due to antigen masking by antibodies or antibody-induced RBC removal, has remained more difficult to define. The ability of anti-Duffy to induce RBC removal, antigen loss, and AMIS at lower doses, suggests that for some monoclonal antibodies, a combination of RBC clearance and antigen modulation may be responsible for AMIS. In contrast, anti-HEL antibodies induce very little, if any, HOD RBC clearance,39 a finding that is consistent with the lack of any detectable change in CD4 T-cell proliferation after anti-HEL engagement. Thus, antibody engagement can have diverse consequences, from antigen removal to RBC clearance, which may affect the likelihood of immune augmentation or AMIS after alloantigen exposure in the presence of an anti-RBC antibody.
Distinct rates of HOD RBC clearance and antigen modulation may account for the differential impact of anti-Duffy on HOD RBC–induced IgM and IgG antibody formation. Given the role of marginal zone (MZ) B cells and MZ constituents in general in early antibody formation,49,60,71 antibody-mediated redirection of HOD RBCs to other immune compartments may reduce the availability of antigen for MZ B-cell–mediated IgM production. In contrast, given the ability of anti-Duffy to both increase HOD RBC antigen presenting cell (APC) uptake and enhance CD4 T-cell proliferation, anti-Duffy–mediated delivery of HOD RBCs to APCs likely facilitates CD4 T-cell proliferation. However, for enhanced CD4 T-cell proliferation to facilitate HOD RBC–induced IgG antibody production, sufficient antigen must be available for B cells to acquire and then present antigen to CD4 T cells. When lower HOD RBC challenge doses are used, increased RBC clearance and enhanced antigen loss may combine to reduce antigen availability below a threshold required for efficient B-cell uptake and subsequent presentation for CD4 T-cell help. In contrast, when higher challenge doses are used, reduced overall RBC clearance coupled with a relative persistence of antigen results in an increased level of circulating HOD RBCs with antigen, allowing B cells to acquire antigen and receive CD4 T-cell help. Consistent with this, inclusion of anti-HEL antibodies resulted in rapid antigen loss and switched the augmented immune response to AMIS. Because no difference in CD4 T-cell activation was observed in the presence of anti-HEL antibodies, these results demonstrate that the CD4 T-cell response, which is often critical for an antibody response, does not necessarily correlate with AMIS. Instead, removal of antigen availability to B cells likely represents a key component of successful AMIS.
Engagement of HOD RBCs by FcγRs not only induces antigen loss but also facilitates CD4 T-cell proliferation. Whether enhanced CD4 T-cell proliferation in this setting reflects direct antigen removal, engagement of immune complexes that may form as a result of antigen removal, or an entirely different process altogether, remains unknown. Intriguingly, FcγR inhibition using 2.4G2 reduced anti-Duffy–mediated clearance but largely failed to affect anti-Duffy–induced antigen loss. Although other approaches, such as Fab generation or antibody deglycosylation, could be used to also evaluate the Fc domain requirements,43,72 the outcomes of FcγR inhibition contrasted those observed in FcγR KO mice. Because 2.4G2 inhibits FcγRs II and III, whereas common γ-chain KOs are deficient in the activity of FcγRs I, III, and IV,73,74 differences in outcomes may reflect distinct FcγRs required for antibody-induce RBC clearance vs modulation. However, simple differences in the sensitivity of HOD RBC clearance and antigen modulation to FcγR inhibition may also account for these differences. Future studies will be needed to define the mechanisms whereby FcγRs remove antigen from the RBC surface.
The ability of antibodies to induce antigen loss instead of RBC removal may result from competing signals that occur after antibody engagement. FcγR recognition of antibody-bound HOD RBCs can promote phagocytosis, whereas competing signals, such as CD47-SIRPα, may inhibit RBC removal. For some RBCs, antigen removal in the absence of RBC phagocytosis is favored, resulting in HOD RBCs with reduced antigen levels; for others, phagocytosis ensues. Although antigen loss may protect HOD RBCs from further antibody-mediated clearance or B-cell recognition, antigen removed from the RBC surface by FcγR positive cells may also facilitate immune cell activation and enhanced CD4 T-cell proliferation. In addition to features of the target antigen itself, key properties of antibodies, including their overall affinity for antigen and glycosylation status, which have been shown to affect overall antibody activity,37,75-77 may contribute to their ability to induce antigen removal and therefore AMIS. Regardless of what antibody or target antigen features dictate their ability to induce AMIS, selecting antibodies that possess the ability to rapidly remove antigen, in the presence or absence of RBC clearance, may be important when seeking possible AMIS candidates.
As with any study, the present approach is not without limitations. First and foremost, this is a model system that may or may not recapitulate critical features of RBC alloimmunization observed clinically. Unfortunately, a model of RhD-induced alloimmunization is not currently available. Thus, whether the present system models RhD is unknown. However, RhIg has been shown to cause both RBC clearance and antigen loss in patients,21,37 suggesting that outcomes similar to that observed for anti-Duffy in this study may be relevant to RhD. Similarly, although current guidelines suggest that RhIg should be operative over a broad range of challenge doses, studies deliberately examining similar concentration ranges clinically in parallel as used in this study are lacking. Because HDFN can result from alloimmunization to a variety of target antigens, dissecting key features of AMIS that are common or distinct after engagement of unique RBC alloantigens will be important when developing formularies to extend the use of AMIS beyond RhD. Furthermore, because the need to prevent alloimmunization is not limited to RBCs, these results may facilitate the development of AMIS for analogous conditions, such neonatal alloimmune thrombocytopenia.78-80 Therefore, although future studies and additional models will be needed to define which features of AMIS may be generalizable among different antigens, the general principles whereby AMIS occurs presented here may provide important insight into key antibody activities needed for successful AMIS formularies.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute, National Institutes of Health grants 5K12HL141953-05 (R.P.J), R01HL154034 (C.M.A.), and P01HL132819, R01HL1335575, and R01HL165975 (S.R.S.).
Authorship
Contribution: R.P.J., J.W.L.A., C.M.A., J.E.H., and S.R.S. conceived the study; K.R.P., D.A., D.M., S.-C.W., P.E.Z., C.L.M., and S.C. provided critical experimental support and reagents; C.J.L., S.C.E., J.D.R., R.M.F., C.D.J., J.P.M., L.C., J.E.H., and K.E.H. provided feedback that helped frame key aspects of how the study was designed, conducted, and ultimately how the data were interpreted; and R.P.J. and S.R.S. wrote the manuscript, which was additionally commented on, and edited by the remaining authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Connie M. Arthur, Joint Program in Transfusion Medicine, Brigham and Women’s Hospital, Harvard Medical School, 630D New Research Bldg, 77 Ave Louis Pasteur, Boston, MA 02115; e-mail: cmarthur@bwh.harvard.edu; and Sean R. Stowell, Joint Program in Transfusion Medicine, Brigham and Women’s Hospital, Harvard Medical School, 630E New Research Bldg, 77 Ave Louis Pasteur, Boston, MA 02115; e-mail: srstowell@bwh.harvard.edu.
References
Author notes
Data are available on request from the corresponding authors, Connie M. Arthur (cmarthur@bwh.harvard.edu) and Sean R. Stowell (srstowell@bwh.harvard.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal