In this issue of Blood, Umino and colleagues1 reveal the intricate immune interactions that contribute to chronic graft-versus-host disease (cGVHD) after allogeneic hematopoietic cell transplantation (HCT) by identifying drivers of antibody production, specifically, the importance of distinct HLA class II alleles in minor histocompatibility antigen (mHA)-driven alloimmunity by H-Y antigens in sex-mismatched HCT.
Female donor-to-male recipient (F-to-M) HCT has a higher risk of GVHD, and this observation has served as a foundation for understanding the role of mHA disparity in pathogenic alloimmunity after HCT by studying the H-Y antigens.2 Despite these advances, the direct downstream effects leading to organ damage and morbidity from cGVHD are not well understood.
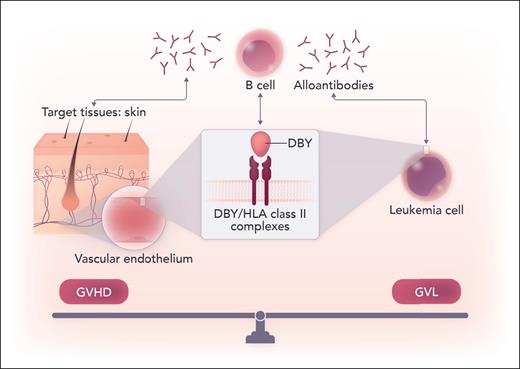
Umino et al evaluated HCT patient plasma, skin biopsies, and leukemia cells to better understand effectors of humoral alloimmunity in cGVHD. Utilizing a national HCT registry of HLA identical transplants, 768 patients who had F-to-M HCT were identified. Using plasma samples, the authors found that specific HLA class II alleles influenced cGVHD risk: HLA-DRB1∗15:02 had the highest risk, and HLA-DRB1∗09:01 appeared protective. Interestingly, these groups had no difference in preceding acute GVHD, suggesting an independent mechanism involved in acute GVHD. The reverse direction (M-to-F) HCT patients also showed HLA-DRB1 allele associations with cGVHD (∗11:01 with increased risk, ∗08:03 with decreased risk) and same-sex (M-to-M) cohorts showed no effect of specific HLA-DR alleles on cGVHD. The cGVHD risk associated with these specific HLA class II molecules appeared directly related to their ability to form complexes with H-Y antigens that allow for transport and presentation of the H-Y antigen on the cell surface, with strongest expression of the H-Y antigen DBY. These complexes were found expressed in dermal vascular endothelium of cGVHD-involved skin, with surrounding myofibroblast markers and tissue fibrosis. Alloantibodies against these complexes triggered complement-dependent cytotoxicity leading to cell death. Although the association of H-Y antigens, and specifically DBY, with development of cGVHD have been previously reported,2,3 this study describes the expression and importance of specific HLA class II/DBY complexes in antibody mediated-cGVHD (see figure). Furthermore, the presence of antibodies against these HLA class II/DBY complexes in patient plasma 3 months post-HCT led to more than double the risk of cGVHD at 1 year, suggesting that these can serve as predictive biomarkers of disease as well as targets for early intervention or prevention.
DBY/HLA class II complexes were found to be expressed on the surface of dermal vascular endothelium as well as leukemia blasts. The downstream humoral responses and production of alloantibodies against these complexes caused cytotoxicity in the skin but also against leukemia, illustrating a potentially targetable common thread between the fine balance of GVHD and GVL. Professional illustration by Somersault18:24.
DBY/HLA class II complexes were found to be expressed on the surface of dermal vascular endothelium as well as leukemia blasts. The downstream humoral responses and production of alloantibodies against these complexes caused cytotoxicity in the skin but also against leukemia, illustrating a potentially targetable common thread between the fine balance of GVHD and GVL. Professional illustration by Somersault18:24.
Related to its cGVHD association, mHA disparities and H-Y antigen detection are associated with durable post-HCT disease remission (ie, lower risk of relapse).2,3 Bridging these findings to the desired graft-versus-leukemia (GVL) effect, the authors also demonstrated expression of these HLA class II/DBY complexes on male patient leukemia cells, which are thus beneficial for the GVL effect.
Although the role of B-cell dysregulation and hyperresponsiveness with antibody production in the pathogenesis of chronic GVHD is now widely recognized,4,5 the exact downstream immune interactions that result in sustained tissue injury are not fully elucidated. B-cell targeting as both a prophylactic and therapeutic strategy for GVHD has also been studied extensively with mixed results,6 illustrating that although B cells play an important role, B-cell depletion alone is not sufficient to prevent or eliminate GVHD. Though this study focused on humoral responses, others have reported a coordinated and synergistic role of specific T-cell populations in DBY-associated immune responses post-HCT,7,8 which suggests the need for combinatorial B- and T-cell-targeted approaches in cGVHD.
This study introduces important novel findings with potential translational implications for risk stratification, predictive biomarkers, and therapeutic targeting. Can we leverage HLA class II mismatching,9 especially given the increasing use of posttransplant cyclophosphamide prophylaxis? Or by knowing the specific HLA class II allele, can clinicians be on alert to closely monitor plasma antibody levels for early detection of cGVHD after sex-mismatched HCT? Should plasma antibody levels be measured routinely post-HCT to detect disease onset and trended to predict response to treatment? These open questions will require further study. Furthermore, the fibrotic manifestations of cGVHD have been some of the most challenging to control and reverse.10 Likewise, the organ predilection of cGVHD is unpredictable. The reported detection of DBY/HLA-DR complexes in dermal vascular endothelium in this study with myofibroblast activation and fibrosis provides an identifiable and potentially intervenable risk factor for fibrotic disease. It would also be worthwhile to better define other organ distributions of these expressed complexes (eg, endothelial cells of the lungs, liver, or oral mucosa) in future studies to better understand if this is a universal systemically targetable mechanism or an organ-specific finding.
This elegant work expands the mechanistic understanding of alloimmunity and the enigma of cGVHD pathogenesis, while introducing an opportunity for both alloantibody-based risk stratification and therapeutic targeting for a patient-tailored approach.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal