In this issue of Blood, Paiva et al1 report that serial monitoring of measurable residual disease (MRD) during maintenance treatment of multiple myeloma (MM) captures dynamic conversion between negative (MRD–) and positive (MRD+) states and robustly anticipates progression-free survival (PFS). They show the feasibility of longer-term MRD monitoring and provide a rationale for trials that investigate intervention before frank relapse.
In 2016, the International Myeloma Working Group (IMWG) published recommendations for capturing the MRD status of patients with MM. This was needed because the previous best outcomes of “complete response” and “stringent complete response” were deemed insufficiently sensitive to reveal the depth and heterogeneity of response to more effective MM treatments.2 These MRD methods include advanced imaging techniques and next-generation sequencing and flow cytometry that can detect a single myeloma cell of 105 to 106 bone marrow cells. Outside of the 2016 IMWG report—but equally exciting—are mass spectrometry assays that detect monoclonal proteins at much lower concentrations than immunofixation; these assays are performed on peripheral blood, so they are more comfortable for patients than bone marrow tests and lend themselves better to longitudinal monitoring.3
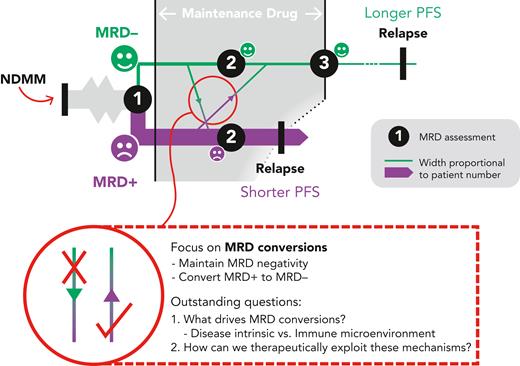
MRD– status after induction/consolidation and early in maintenance treatment strongly predicts PFS in transplant eligible patients4 and is similarly informative during continuous therapy for the transplant-ineligible cohort, where—additionally—sustained MRD negativity correlates with longer PFS.5 A recent meta-analysis found that, regardless of methodology or sensitivity threshold, MRD negativity portends better outcomes; and that the more sensitive the method, the longer the PFS and overall survival predicted by an MRD– result.6 MRD acts as a surrogate outcome measure in clinical trials, providing an earlier read out with the potential to accelerate drug development. More directly, we can envisage its routine use in the myeloma clinic, where MRD status could guide prognostication and in due course therapy decisions (see figure).
A model for MRD monitoring. MRD status is assessed following induction/consolidation. The key aim of the maintenance phase is to maximize the chance of MRD negativity to lengthen PFS. NDMM, newly-diagnosed MM.
A model for MRD monitoring. MRD status is assessed following induction/consolidation. The key aim of the maintenance phase is to maximize the chance of MRD negativity to lengthen PFS. NDMM, newly-diagnosed MM.
To address this latter application, and bring MRD monitoring closer to the clinic, Paiva et al have assessed the practicality of serial MRD monitoring and investigated the dynamics of MRD during the maintenance phase of treatment—a hitherto under-explored arena. They pooled next-generation flow MRD data from 1362 patients recruited to the TOURMALINE-MM3 and -MM4 (Study of Oral Ixazomib Citrate [MLN9708] Maintenance Therapy in Participants With Multiple Myeloma) trials, which compared 2-year fixed-duration ixazomib (a proteasome inhibitor) against placebo as maintenance therapy in the post-autologous stem cell transplant (ASCT) (-MM3) and post-induction in transplant-ineligible patients (-MM4) settings.7,8 The presence of a placebo (monitoring) group sheds light on the natural history of MRD dynamics—a highly valuable insight, given maintenance therapy is now routine practice.
In the first instance, Paiva et al sought to reproduce the findings of others; namely, that a single MRD reading after completion of induction is predictive of PFS. Sure enough, patients who were MRD– at this time point had a median PFS of 38.6 months, vs 15.6 months for those who were MRD+. The benefit of the MRD– status held regardless of baseline factors such as prior treatment or presence of high-risk cytogenetics. In addition, the investigators were able to use the serial nature of their datasets to describe the dynamic nature of MRD—yielding arguably the most interesting findings of the study. Over the course of evaluation, 9.5% of patients converted from MRD– to MRD+ and, in so doing, acquired a poor prognosis equivalent to those who had remained MRD+ throughout (a 2-year PFS rate of approximately 30%). Conversely, 5.1% of patients converted from MRD+ to MRD– and,in so doing, adopted a PFS akin to those who had sustained MRD negativity (2-year PFS of approximately 75%). This shows how serial MRD can provide a more accurate prognosis than a single time point measurement, whereas the results themselves provide motivation for the design of future studies to improve outcomes in patients converting to MRD+.
Naturally, Paiva et al then assessed whether ixazomib (the investigational agent) modified the dynamics of MRD. Ixazomib was associated with a longer median PFS than placebo only in MRD+ patients (18.8 months vs 11.6 months). However, this could not be explained by rates of conversion from MRD+ to MRD–, which were equivalent between the two treatment groups. The investigators postulate that ixazomib cannot eradicate residual clones but simply keeps them in check. Monitoring MRD dynamics can potentially shed light on the activity of maintenance agents in either inducing or maintaining MRD negativity.
This work from Paiva et al inspires many questions that should be addressed in prospective studies. Can we shorten maintenance duration in patients who have sustained MRD negativity, thus sparing them the burden of ongoing medication? Equally, should we escalate therapy for MRD+ status to convert to MRD–? Can blood-based MRD assays facilitate earlier detection of change in MRD? How do these marrow and blood MRD dynamics correlate with imaging? Can we predict which patients will convert from MRD– to MRD+?
This work and other studies also raise new questions in the biology of MRD dynamics and the role of disease-intrinsic factors and MM-immune interactions in the microenvironment. With increasing treatment options made available for MM, dissecting mechanisms of MRD switch and persistent MRD positivity will inform the rational design of treatment strategies that will hopefully improve patient outcomes in MM.
Conflict-of-interest disclosure: K.R. received advisory board and speaker fees from AbbVie, Adaptive Biotechnologies, Amgen, Celgene (BMS), EUSA Pharma, GSK, Janssen, Karyopharm, Oncopeptides, Pfizer, Sanofi, and Takeda; and research support from Amgen, BMS (Celgene), GSK, Janssen, and Takeda. E.W. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal