In this issue of Blood, Schmoellerl et al leveraged both human and murine models of ecotropic virus integration site-1 (EVI1)-driven acute myeloid leukemia (AML), on which they performed functional genome-wide genetic screens to identify genes that are essential to maintain the leukemic cells of this aggressive subtype of AML cells.1 They identified ERG as a uniquely conserved direct transcriptional target of EVI1, which strongly contributes to enhanced cell survival and differentiation blockage in these AML cells.
AML is an aggressive malignancy driven by genetic alterations in hematopoietic stem and progenitor cells that frequently tlead to aberrant expression and/or activation of transcription factors or coregulators. Although chemotherapy for AML has progressed over the past 40 years, several molecular subgroups, including leukemia overexpressing the EVI1 zinc finger transcription factor, remain associated with poor clinical outcome. EVI1 is encoded at the MECOM locus at 3q26.2, which is rearranged by several types of chromosomal alterations leading to EVI1 overexpression (EVI1High). This overexpression can result from the relocation of enhancers, including GATA binding protein 2 (GATA2) enhancer in inv(3)/t(3;3)(q21q26)2 and MYC super-enhancer in t(3;8)(q26;q24) close to the EVI1 gene, or from expression of fusion oncoproteins [eg, runt-related transcription factor 1 (RUNX1)-EVI1 in t(3;21) (q26;q22), ETV6-EVI1 in t(3;12)(q26;p13). Overall, the consequences of chromosomal rearrangements on EVI1 transcription have been well characterized in recent years, and only a few unexplained cases remain. However, the gene regulatory program downstream of EVI1 is less well understood.
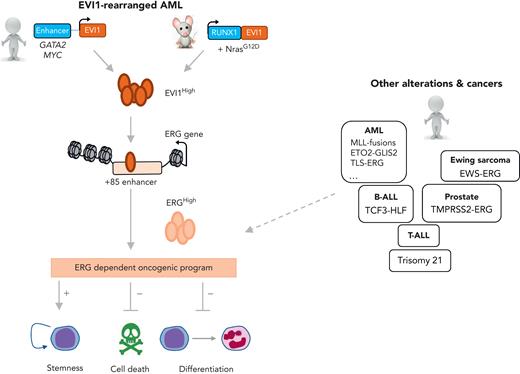
Schmoellerl et al created a novel murine transgenic model of RUNX1-EVI1 expression combined with a NrasG12D mutation recurrently observed in EVI1High AML patients (see figure). To identify genes that are transcriptionally controlled by EVI1, expression analyses were performed in this murine model and in the human HNT34 cell line carrying t(3;3)(q21;q26) after EVI1 knockdown. In these 2 contexts, a common core set of genes were positively controlled by EVI1, including transcriptional factors/coregulators (eg, BCL11A, CBX6, ERG, HHEX, LYL1), which were also enriched in EVI1-rearranged AML patients. At the loci of these gene targets in the RUNX1-EVI1 murine model, EVI1 enforced higher chromatin accessibility. To establish functional EVI1-specific dependencies, the authors performed genome-wide gene inactivation screens in human and murine EVI1-dependent models using a CRISPR (for clustered regularly inter-spaced short palindromic repeats)/Cas9 (for CRISPR-associated) approach and compared their hits with those obtained in other published screens. A strength of the study is that it integrated data from expression analyses and functional screens, resulting in the identification of ERG as the sole candidate that is both directly transcriptionally regulated by EVI1 and essential for the survival of EVI1-driven AML in human and murine models. Validation of these data through specific ERG knockdown showed that ERG contributes to myeloid differentiation blockage, the anti-apoptotic cell survival effect, and leukemia maintenance in both of their models. An elegant rescue experiment in the RUNX1-EVI1 murine model revealed that ERG ectopic expression was sufficient to overcome the effect of EVI1 knockdown on cell survival and restore expression of 34% of EVI1 target genes (eg, MYC, KIT), indicating strongly that ERG mediates a significant part of the EVI1 gene regulatory network involved in leukemic cell survival, proliferation, and stemness maintenance.
Ecotropic virus integration site-1 (EVI1)-rearranged acute myeloid leukemia (AML) joins the club of ERG-dependent alterations and cancers. In this issue of Blood, Schmoellerl et al have studied 2 EVI1-driven human and murine leukemia models (EVI1High). They showed that EVI1 binds and regulates the open chromatin state at the ERG gene, leading to high expression of this E-twenty six (ETS) transcription factor. ERG enforces an oncogenic program, including self-renewal and survival properties associated with hematopoietic differentiation blockade. Some of the other genetic alterations and human cancers associated with high ERG expression or dependency are indicated on the right side, including different molecular subgroups of AML, B-cell acute lymphoblastic leukemia (B-ALL), T-cell acute lymphoblastic leukemia (T-ALL), Ewing sarcoma, and prostate cancer. MLL, mixed lineage leukemia; RUNX1, runt-related transcription factor 1.
Ecotropic virus integration site-1 (EVI1)-rearranged acute myeloid leukemia (AML) joins the club of ERG-dependent alterations and cancers. In this issue of Blood, Schmoellerl et al have studied 2 EVI1-driven human and murine leukemia models (EVI1High). They showed that EVI1 binds and regulates the open chromatin state at the ERG gene, leading to high expression of this E-twenty six (ETS) transcription factor. ERG enforces an oncogenic program, including self-renewal and survival properties associated with hematopoietic differentiation blockade. Some of the other genetic alterations and human cancers associated with high ERG expression or dependency are indicated on the right side, including different molecular subgroups of AML, B-cell acute lymphoblastic leukemia (B-ALL), T-cell acute lymphoblastic leukemia (T-ALL), Ewing sarcoma, and prostate cancer. MLL, mixed lineage leukemia; RUNX1, runt-related transcription factor 1.
By demonstrating that EVI1High AML cells depend on ERG, these data extend the central oncogenic role of this E-twenty six (ETS)-family transcription factor in human acute leukemia associated with poor prognosis. Indeed, EVI1High ERGHigh expression is a distinctive feature of an aggressive subset of mixed lineage leukemia (MLL)-rearranged AML.3 An independent study on MLL-eleven nineteen leukemia (ENL)+ cells found that EVI1High cells were more dependent on ERG than EVI1Low cells.4 ERG, encoded on chromosome 21, also has functional relevance for ETO2-GLIS2+ AML5 as well as acute lymphoblastic leukemia (ALL), including T-cell ALL6 and TCF3-HLF+ B-cell ALL,7 but whether EVI1 is also involved in these types of leukemia remains to be studied.
Mechanistically, EVI1 was shown here to bind and regulate the open chromatin state of the ERG locus at a +85 enhancer, a regulatory element previously shown to be controlled by a transcriptional complex referred to as the heptad complex,8 including several factors that are important for normal hematopoietic stem cell biology and are altered in EVI1- and ERG-dependent AML (eg, RUNX1, GATA2, ETO2). In addition, although EVI1 is often referred to as a repressor interacting with C-terminal binding protein (CtBP), it also positively controls a gene expression program including stemness genes. Therefore, the precise combinatorial interplay between EVI1 and heptad transcription factors, as well as the relative contribution of other cofactors controlling the locus-specific transcriptional activation of this stemness program is an interesting area for future investigation.
These data and other observations also indicate that an interplay occurs between the genetic-context or the cell-context and EVI1 activity. Here, Schmoellerl et al found that not all of the EVI1-rearranged AML cell lines are equally sensitive to EVI1 knockdown. Specifically, HNT34 was highly sensitive, whereas MOLM1 and Kasumi3 were less affected. Interestingly, only HNT34 cells also presented an additional SF3B1 mutation that controls the expression of an EVI1 isoform with a 6–amino-acid insertion close to the zinc fingers and an enhanced self-renewal stimulation property.9 Whether this isoform is responsible for enhanced ERG expression is unknown. Regarding the importance of the cellular context, EVI1 and ERG both show decreasing expression upon differentiation of normal hematopoietic stem cells. Still unknown is whether the cell of origin in which the EVI1 rearrangement occurs determines the level of ERG expression in the resulting AML, and whether EVI1 and ERG expression levels vary during leukemic evolution. Indeed, ERG could progressively substitute for EVI1 oncogenic function through differentiation or epigenetic drift toward EVI1-independent leukemia in which ERG could enforce leukemia cell survival and maintenance.
Finally, these data provide new perspectives on future development of therapeutics. Indeed, although direct targeting of the driver EVI1 oncogene remains desirable, achieving specific inhibition of ERG activity will most likely be of wider therapeutic interest for several subtypes of aggressive AML, including EVI1High AML. With this goal, ERG pharmacologic inhibitors are being developed, and ERG protein structure–based strategies may also allow for the development of inhibitory peptidomimetic approaches.10
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal