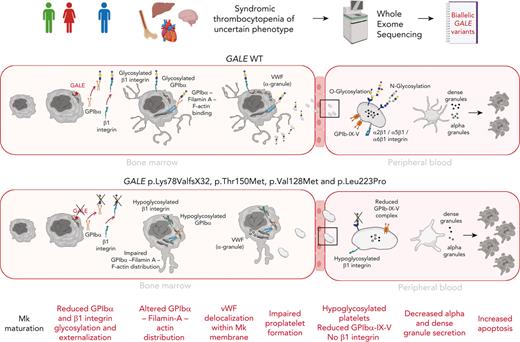
Key Points
GALE is expressed during late-stage megakaryopoiesis, regulating the glycosylation and surface exposure of GPIbα and β1 integrin.
Novel GALE variants cause syndromic macrothrombocytopenia associated with impaired platelet formation and function.
Abstract
Glycosylation is recognized as a key process for proper megakaryopoiesis and platelet formation. The enzyme uridine diphosphate (UDP)-galactose-4-epimerase, encoded by GALE, is involved in galactose metabolism and protein glycosylation. Here, we studied 3 patients from 2 unrelated families who showed lifelong severe thrombocytopenia, bleeding diathesis, mental retardation, mitral valve prolapse, and jaundice. Whole-exome sequencing revealed 4 variants that affect GALE, 3 of those previously unreported (Pedigree A, p.Lys78ValfsX32 and p.Thr150Met; Pedigree B, p.Val128Met; and p.Leu223Pro). Platelet phenotype analysis showed giant and/or grey platelets, impaired platelet aggregation, and severely reduced alpha and dense granule secretion. Enzymatic activity of the UDP-galactose-4-epimerase enzyme was severely decreased in all patients. Immunoblotting of platelet lysates revealed reduced GALE protein levels, a significant decrease in N-acetyl-lactosamine (LacNAc), showing a hypoglycosylation pattern, reduced surface expression of gylcoprotein Ibα-IX-V (GPIbα-IX-V) complex and mature β1 integrin, and increased apoptosis. In vitro studies performed with patients-derived megakaryocytes showed normal ploidy and maturation but decreased proplatelet formation because of the impaired glycosylation of the GPIbα and β1 integrin, and reduced externalization to megakaryocyte and platelet membranes. Altered distribution of filamin A and actin and delocalization of the von Willebrand factor were also shown. Overall, this study expands our knowledge of GALE-related thrombocytopenia and emphasizes the critical role of GALE in the physiological glycosylation of key proteins involved in platelet production and function.
Introduction
Inherited thrombocytopenias (ITs) are a group of rare and heterogeneous platelet disorders characterized by low platelet count, which lead to variable increased risk of bleeding depending on the degree of thrombocytopenia and the concomitant association of significant platelet dysfunction.1 It is currently well-established that many ITs evolve with the development of other serious congenital defects that affect different organs or additional diseases including hematological malignancies, bone marrow failure, and/or nonhematological defects.2,3 ITs are caused by genetic alterations in megakaryopoiesis-related genes. Early stages of megakaryopoiesis that lead to megakaryocyte (MK) differentiation can be disrupted by alterations in genes that have major roles in the fine-tuning of hematopoietic stem cell homeostasis or regulating the thrombopoietin (TPO) signaling axis. The late stages of the megakaryopoiesis, which include MK maturation and migration, proplatelet formation, and platelet release into the bloodstream, can be impaired by not only rare molecular changes in genes encoding for critical transcriptional factors, vesicle trafficking, or biochemical signals, but also cytoskeletal proteins, platelet surface glycoproteins (GP), ion channels, metabolic genes, and genes affecting platelet survival.4-10 Protein glycosylation and linked sialylation are common and complex post-translational modifications, with a critical role in different biological processes such as protein clearance and degradation, immunity, thrombosis and, hemostasis.11 These modifications are also important in megakaryopoiesis12 because mice with genetic deficiencies in glycosylation/sialylation present thrombocytopenia.11,13,14 The use of whole-exome sequencing (WES) unveiled rare cases of syndromic ITs caused by recessive variants in crucial genes for regulating sialylation, such as GNE, involved in the sialic acid synthesis,15,16 the sialic transporter gene SLC35A1,17,18 or genes implicated in glycosylation, such as GALE, which encodes the uridine diphosphate (UDP)-galactose-4-epimerase.19-21 Here, we report 3 patients from 2 unrelated families who showed lifelong syndromic thrombocytopenia and platelet dysfunction caused by compound heterozygosity of 4 rare GALE variants, 3 of those previously unreported. Our data further support the key role of GALE in protein glycosylation. We unveiled GALE variants driving novel mechanisms of pathological thrombopoiesis that consists of impaired glycosylation and externalization of GPIbα and β1 integrin during the late stages of megakaryopoiesis, which lead to altered proplatelet formation by disrupting the filamin A and actin cytoskeleton and to the production of platelets with impaired morphology, function, and viability.
Patients, materials, and methods
Additional methodological details can be found in the supplemental Material, available on the Blood website.
Patients, platelet phenotyping, whole-exome sequencing, and variant interpretation
Three patients from 2 unrelated and nonconsanguineous families presenting with syndromic thrombocytopenia of unknown origin were investigated (Figure 1A). Patients’ general features and characteristics are summarized in Table 1. Venous blood samples were drawn into EDTA or sodium citrate according to the different studies. Assessment of platelet phenotype, WES, and variant classification were performed in 4 members of pedigree A and 3 members of pedigree B (Figure 1A), as previously described.22-24
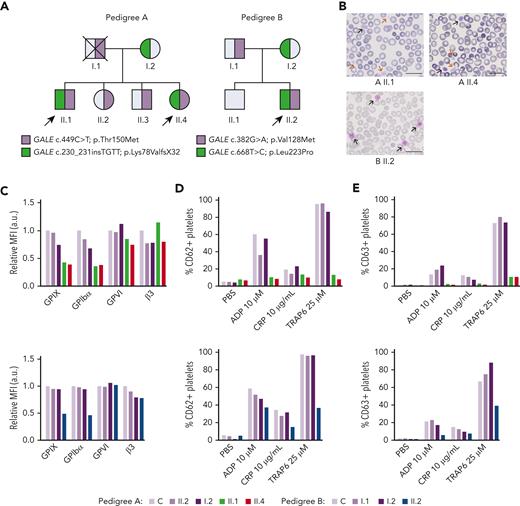
Patients carrying GALE variants showed giant and/or grey platelets in blood films and impaired granules secretion. (A) Family segregation of the variants affecting GALE identified by whole-exome sequencing. Probands are indicated with black arrows. Partially filled symbols in purple or green represent heterozygous status for the indicated GALE variant. (B) Representative peripheral blood films from probands of the 2 pedigrees after May-Grunwald Giemsa staining (×100). Red and black arrows indicate grey and giant platelets, respectively. (C) GPs expression in control and patients, assessed by flow cytometry with fluorescent-labeled antibodies anti-CD42a, CD42b, GPVI, and CD61. The MFI of each antibody, standardized by size (FSC) and relative to the control sample, is represented. (D-E) Platelets from healthy control and family members were stimulated with agonists in the presence of appropriate monoclonal antibodies to assess (D) alpha granules secretion (anti-CD62P), (E) dense granules secretion (anti-CD63) evaluated by flow cytometry. Plots show the percentage of positive platelets. a.u., arbitrary units; ADP, Adenosine diphosphate; CRP; collagen-related peptide; MFI, mean fluorescence intensity; PBS; phosphate-buffered saline; TRAP; thrombin receptor activator peptide.
Patients carrying GALE variants showed giant and/or grey platelets in blood films and impaired granules secretion. (A) Family segregation of the variants affecting GALE identified by whole-exome sequencing. Probands are indicated with black arrows. Partially filled symbols in purple or green represent heterozygous status for the indicated GALE variant. (B) Representative peripheral blood films from probands of the 2 pedigrees after May-Grunwald Giemsa staining (×100). Red and black arrows indicate grey and giant platelets, respectively. (C) GPs expression in control and patients, assessed by flow cytometry with fluorescent-labeled antibodies anti-CD42a, CD42b, GPVI, and CD61. The MFI of each antibody, standardized by size (FSC) and relative to the control sample, is represented. (D-E) Platelets from healthy control and family members were stimulated with agonists in the presence of appropriate monoclonal antibodies to assess (D) alpha granules secretion (anti-CD62P), (E) dense granules secretion (anti-CD63) evaluated by flow cytometry. Plots show the percentage of positive platelets. a.u., arbitrary units; ADP, Adenosine diphosphate; CRP; collagen-related peptide; MFI, mean fluorescence intensity; PBS; phosphate-buffered saline; TRAP; thrombin receptor activator peptide.
Clinical and laboratory characteristics of 3 patients from the 2 unrelated families
| Patient . | Pedigree A. II.1 . | Pedigree A. II.4 . | Pedigree B. II.2 . |
|---|---|---|---|
| Age, (y) | 45 | 37 | 38 |
| Sex | Male | Female | Male |
| Bleeding score, ISTH-BAT | 8 | 10 | 6 |
| Bleeding symptoms | Mucocutaneous (1), epistaxis (2), minor wounds (2) conjunctival and vitrectomy (3) | Mucocutaneous (1), epistaxis (2), dental extraction (2), menorrhagia (2), surgical hemostasis after ovarian cyst (3) | Mucocutaneous (2) and gastrointestinal (4) |
| Hemoglobin level, g/dL (N, 12-16 g/dL) | 12.1 | 11.5 | 13 |
| White blood cells (N, 3.8 × 109/L to 11 × 109/L) | 9.9 × 109/L | 3.5 × 109/L | 8.8 × 109/L |
| Neutrophils (N, 1.4 × 109/L to 6.5 × 109/L); | 5.1 × 109/L | 1.6 × 109/L | 5.6 × 109/L |
| Platelet count (N, 150 × 109/L to 450 × 109/L) | 12.7 × 109/L | 5.3 × 109/L | 17 × 109/L |
| MPV (fL) (N, 7.2-11.1 fL) | 17.4 | 19.2 | 20 |
| IPF, % (N, 1.1%-6.1%) | 76 | NA | 43 |
| Blood film (MPD) | Large: 14% Giant: 30% Grey: 54% | Large: 24% Giant: 48% Grey: 24% | Large: 16% Giant: 72% Grey: 8% |
| Bone marrow aspirate | Erythroid hyperplasia and increased MKs | Erythroid hyperplasia and increased MKs | Not done |
| Previous diagnosis | Immune thrombocytopenia | Immune thrombocytopenia | DiGeorge syndrome Grey platelet syndrome |
| Treatments | Corticosteroids Immunoglobulins Splenectomy | Corticosteroids Immunoglobulins | No |
| Red blood cells transfusions | Yes | Yes | Yes |
| Platelet transfusions | Yes | Yes | Yes |
| Additional symptoms: | Yes | Yes | Yes |
| Neurological | Mental retardation | Mental retardation | Mental retardation |
| Cardiovascular | Mitral valve prolapse and mitral insufficiency | Mitral valve prolapse and mitral insufficiency | Mitral valve prolapse and mitral insufficiency |
| Ophthalmological | Retinal disease Cataracts | Retinal disease Cataracts | No |
| Orthopedic | No | Hip dysplasia | Equine foot |
| Hepatic | Jaundice Hepatomegaly | Jaundice Hepatomegaly | Jaundice Hepatomegaly |
| The parents and other relatives in both families were asymptomatic and had normal platelet count and volume. | |||
| Patient . | Pedigree A. II.1 . | Pedigree A. II.4 . | Pedigree B. II.2 . |
|---|---|---|---|
| Age, (y) | 45 | 37 | 38 |
| Sex | Male | Female | Male |
| Bleeding score, ISTH-BAT | 8 | 10 | 6 |
| Bleeding symptoms | Mucocutaneous (1), epistaxis (2), minor wounds (2) conjunctival and vitrectomy (3) | Mucocutaneous (1), epistaxis (2), dental extraction (2), menorrhagia (2), surgical hemostasis after ovarian cyst (3) | Mucocutaneous (2) and gastrointestinal (4) |
| Hemoglobin level, g/dL (N, 12-16 g/dL) | 12.1 | 11.5 | 13 |
| White blood cells (N, 3.8 × 109/L to 11 × 109/L) | 9.9 × 109/L | 3.5 × 109/L | 8.8 × 109/L |
| Neutrophils (N, 1.4 × 109/L to 6.5 × 109/L); | 5.1 × 109/L | 1.6 × 109/L | 5.6 × 109/L |
| Platelet count (N, 150 × 109/L to 450 × 109/L) | 12.7 × 109/L | 5.3 × 109/L | 17 × 109/L |
| MPV (fL) (N, 7.2-11.1 fL) | 17.4 | 19.2 | 20 |
| IPF, % (N, 1.1%-6.1%) | 76 | NA | 43 |
| Blood film (MPD) | Large: 14% Giant: 30% Grey: 54% | Large: 24% Giant: 48% Grey: 24% | Large: 16% Giant: 72% Grey: 8% |
| Bone marrow aspirate | Erythroid hyperplasia and increased MKs | Erythroid hyperplasia and increased MKs | Not done |
| Previous diagnosis | Immune thrombocytopenia | Immune thrombocytopenia | DiGeorge syndrome Grey platelet syndrome |
| Treatments | Corticosteroids Immunoglobulins Splenectomy | Corticosteroids Immunoglobulins | No |
| Red blood cells transfusions | Yes | Yes | Yes |
| Platelet transfusions | Yes | Yes | Yes |
| Additional symptoms: | Yes | Yes | Yes |
| Neurological | Mental retardation | Mental retardation | Mental retardation |
| Cardiovascular | Mitral valve prolapse and mitral insufficiency | Mitral valve prolapse and mitral insufficiency | Mitral valve prolapse and mitral insufficiency |
| Ophthalmological | Retinal disease Cataracts | Retinal disease Cataracts | No |
| Orthopedic | No | Hip dysplasia | Equine foot |
| Hepatic | Jaundice Hepatomegaly | Jaundice Hepatomegaly | Jaundice Hepatomegaly |
| The parents and other relatives in both families were asymptomatic and had normal platelet count and volume. | |||
Bleeding symptoms were scored (n) by the ISTH-BAT questionnaire.
IPF, immature platelet fraction; ISTH-BAT, International Society on Thrombosis and Haemostasis bleeding assessment tool; MPD, mean platelet diameter; MPV, mean platelet volume; N, normal; NA. not available.
UDP-galactose-4-epimerase activity and immunoblotting
The UDP-galactose-4-epimerase activity was measured in dried blood spots by high-performance liquid chromatography with tandem mass spectrometry, adapted from previous publications.25 Protein levels were assessed in platelet lysates by standard immunoblotting and probed with selected antibodies. Protein levels in MK were evaluated by automated capillary-based Western blotting.
Human megakaryocyte differentiation
CD45+ or CD34+ cells were separated by immunomagnetic bead selection (Miltenyi Biotec, Bologna, Italy) and cultured in the presence of TPO, as previously described.26,27 At the end of the culture (14th day), polyploidization, maturation, apoptosis, and total and surface (permeabilized and nonpermeabilized cells, respectively) protein expression were analyzed by flow cytometry.26,27 Mature MKs were characterized by microscopy using a Leica TCS-SP5 or TCS-SP8 confocal microscope. Images were analyzed using ImageJ software.
Adhesion to extracellular matrices
Control and patient platelets were set in coverslips (1 × 106 total platelets per coverslip) coated with laminin, fibronectin, collagen, or Haemate-P, as described,28 and stained with selective antibodies.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8 Software (GraphPad Software). Differences among groups were tested with t-student, 1- and 2-way analyses of variance, and Tukey’s multiple comparisons test. Statistically significant difference was achieved for P-values <.05.
Ethics statement
The study was abided by the Declaration of Helsinki and approved by the Ethics Committees of the Instituto de Investigación Biomédica de Salamanca (IBSAL) (reference PI5505/2017). All patients, family members, and healthy controls gave written informed consent. Umbilical cord blood units were collected after obtaining the parents’ written informed consent at enrollment in accordance with the ethical committee of the I.R.C.C.S. Policlinico San Matteo foundation of Pavia and the principles of the Declaration of Helsinki.
Results
Clinical presentation of 2 unrelated families
The 3 probands (siblings II.1 & II.4 in pedigree A; II.2 in pedigree B) presented lifelong severe macrothrombocytopenia associated with moderate to severe bleeding tendency and other clinical features such as mental retardation, mitral valve prolapse, and increased bilirubin levels (Figure 1A; Table 1). Proband A.II.1 and B.II.2 displayed 76% and 43% of reticulated platelets, respectively (Table 1). Notably, siblings II.1 & II.4 in pedigree A had been previously misdiagnosed with immune thrombocytopenia, were treated with corticosteroids and immunoglobulins, and lastly underwent splenectomy (A.II.1). In contrast, an IT was suspected in proband II.2 from pedigree B and he was only treated with platelet transfusions before invasive procedures (Table 1).
Assessment of patients’ platelet phenotype
Blood film revealed a significant proportion of enlarged, giant, and/or grey platelets in all probands (Figure 1B; Table 1). Moreover, typical morphological findings of patients undergone splenectomy, such as erythroblast, were observed in the blood film from proband A.II.1. No other relevant morphological abnormalities appeared.
All probands displayed significantly reduced platelet aggregation upon stimulation with several agonists (supplemental Figure 1A; available on Blood website). Flow cytometry analysis showed a comparable expression of GPVI and αIIbβ3 integrin in probands vs nonaffected relatives and healthy control but a significant reduction in the GPIbα and GPIX levels (GPIb-IX-V complex) (50%-70%) (Figure 1C). Furthermore, agonist-induced P–selectin and CD63 exposure were severely impaired (Figures 1D-E). Lastly, patients’ platelets also showed a slightly reduced fibrinogen binding and normal thrombin receptor activating peptide 6 (TRAP-6)–induced platelet spreading on fibrinogen, suggesting substantial preservation of the αIIbβ3 function (supplemental Figures 1B-D).
WES analysis in patients and relatives identifies GALE candidate variants
WES unveiled the presence of compound heterozygous variants in GALE (NM_001127621.2) in all the probands (Figure 1A), according to the recessive inheritance of their disease. Patients from pedigree A (II.1 and II.4) carried the previously reported missense variant c.449C>T [p.Thr150Met],21 which affects exon 5, and the novel insertion c.230_231insTGTT [p.Lys78ValfsX32], which affects exon 3. The proband from pedigree B (II.2) carried the 2 novel missense variants c.382G>A [p.Val128Met] and c.668T>C [p.Leu223Pro] at exons 5 and 7, respectively. Of note, all these mutations are located nearby NAD+ binding site or the substrate-binding site of the GALE-encoded protein, UDP-galactose-4-epimerase (supplemental Figure 2). Considering these data, variants were reclassified as pathogenic variants (supplemental Table 1).
GALE variants lead to reduced levels and impaired enzymatic activity of UDP-galactose-4-epimerase, and increase in platelet apoptosis
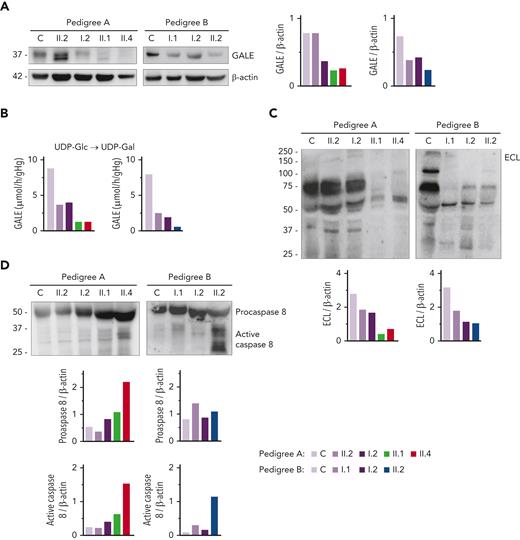
Immunoblotting assays revealed reduced levels of UDP-galactose-4-epimerase (GALE protein) in platelet of patients compared with that of unaffected family members and healthy controls. As shown in Figure 2A, platelets from patients A.II.1 and A.II.4 had a 50% overall expression of GALE in comparison with control platelets, because of the p.Lys78ValfsX32 variant. In addition, samples from patients A.II.1 and A.II.4 presented a tiny protein band with different electrophoretic mobility corresponding to the mutant form of the enzyme (p.Thr150Met). The total expression of the enzyme in the unaffected A.II.2 was similar to that in the healthy control, but 2 bands corresponding to native and mutant (p.Thr150Met) proteins were detectable (Figure 2A). In contrast, A.I.2, the patient’s mother carrying p.Lys78ValfsX32, displayed a 50% reduced expression of the protein, which is consistent with haploinsufficiency (Figure 2A). In pedigree B, platelet from proband II.2 displayed 35% of UDP-galactose-4-epimerase levels vs control platelets, whereas platelets from the unaffected relatives B.I.1 and B.I.2 showed a milder decrease (51% and 65% expression, respectively), suggesting the existence of a protein instability phenomenon, in agreement with the negative effect reported for other mutations.29,30
GALE variants associated with reduced GALE protein levels, impaired enzymatic activity, platelet hypoglycosylation, and increased apoptosis. (A) Western blotting from platelet lysates of patients from both pedigrees, unaffected relatives, and controls. Membranes were blotted with anti-GALE and anti-β-actin as an internal control. A.II.2 presented total levels of GALE protein similar to controls, but 2 GALE bands with different electrophoretic mobility were observed (top, GALE-WT; bottom, GALE p.Thr150Met), whereas A.I.2 displayed a 50% reduction of total GALE protein. Probands from pedigree A (II.1 and II.4) showed reduced protein levels (50%) and a tiny band corresponding to GALE p.Thr150Met. Individuals from pedigree B: I.1, I.2, and II.2 showed reduced total GALE protein levels (51%, 65%, and 35%, respectively) vs control. (B) Enzymatic activity of UDP-galactose-4-epimerase assessed in members of pedigrees A and B. The activity of a healthy control was evaluated in parallel. Probands of both families showed a sharp reduction in the GALE enzymatic activity (A.II.1, 15%; A.II.4, 15%; and B.II.2, 7.5%), whereas heterozygous carriers in both families displayed a moderate reduction (A.II.2, 42%; A.I.1, 45%; B.I.1, 31%; and B.I.2, 24%). (C) To assess the glycosylation profile, platelet lysates were probed with lectin ECL, which specifically binds to LacNAc, a dimer of N-acetyl-glucosamine and galactose molecules. β-actin staining, shown in panel A, was used as an internal control. A sharp decrease in ECL binding was found in all probands, less pronounced in heterozygous carriers of a single GALE variant. (D) Platelet lysates were probed with antibodies against procaspase 8 (56 kDa) (top band) or the active-cleaved form of caspase 8 (bottom bands). β-actin (shown in panel A) was the internal protein control. Proband from pedigree A displayed increased procaspase 8 and active caspase 8 level, whereas in the proband from the pedigree B only the active caspase 8 was increased. Band intensities were quantified by densitometric analysis using the ‘Image J’ software. ECL, Erythrina crista-galli lectin; LacNAc, N-acetyl-lactosamine; UDP-Glc, UDP-glucose; UDP-Gal, UDP-galactose.
GALE variants associated with reduced GALE protein levels, impaired enzymatic activity, platelet hypoglycosylation, and increased apoptosis. (A) Western blotting from platelet lysates of patients from both pedigrees, unaffected relatives, and controls. Membranes were blotted with anti-GALE and anti-β-actin as an internal control. A.II.2 presented total levels of GALE protein similar to controls, but 2 GALE bands with different electrophoretic mobility were observed (top, GALE-WT; bottom, GALE p.Thr150Met), whereas A.I.2 displayed a 50% reduction of total GALE protein. Probands from pedigree A (II.1 and II.4) showed reduced protein levels (50%) and a tiny band corresponding to GALE p.Thr150Met. Individuals from pedigree B: I.1, I.2, and II.2 showed reduced total GALE protein levels (51%, 65%, and 35%, respectively) vs control. (B) Enzymatic activity of UDP-galactose-4-epimerase assessed in members of pedigrees A and B. The activity of a healthy control was evaluated in parallel. Probands of both families showed a sharp reduction in the GALE enzymatic activity (A.II.1, 15%; A.II.4, 15%; and B.II.2, 7.5%), whereas heterozygous carriers in both families displayed a moderate reduction (A.II.2, 42%; A.I.1, 45%; B.I.1, 31%; and B.I.2, 24%). (C) To assess the glycosylation profile, platelet lysates were probed with lectin ECL, which specifically binds to LacNAc, a dimer of N-acetyl-glucosamine and galactose molecules. β-actin staining, shown in panel A, was used as an internal control. A sharp decrease in ECL binding was found in all probands, less pronounced in heterozygous carriers of a single GALE variant. (D) Platelet lysates were probed with antibodies against procaspase 8 (56 kDa) (top band) or the active-cleaved form of caspase 8 (bottom bands). β-actin (shown in panel A) was the internal protein control. Proband from pedigree A displayed increased procaspase 8 and active caspase 8 level, whereas in the proband from the pedigree B only the active caspase 8 was increased. Band intensities were quantified by densitometric analysis using the ‘Image J’ software. ECL, Erythrina crista-galli lectin; LacNAc, N-acetyl-lactosamine; UDP-Glc, UDP-glucose; UDP-Gal, UDP-galactose.
In consequence, all patients exhibited a sharp decrease in the enzymatic activity of the UDP-galactose-4-epimerase, in comparison with healthy controls (A.II.1, 15%; A.II.4, 15%; and B.II.2, 7.5%) (Figure 2B).
Because UDP-galactose-4-epimerase catalyzes the interconversion of UDP-glucose to UDP-galactose and UDP-N-acetyl-glucosamine to UDP-N-acetyl-galactosamine, thus acting on 4 molecules involved in the N-linked and O-linked glycosylation (supplemental Figure 3), we decided to assess the glycosylation pattern in platelet lysates. Immunoblotting assays with lectin ECL, which specifically binds to LacNAc (a dimer of N-acetyl-glucosamine and galactose), showed decreased LacNAc levels in platelets from the patients (A.II.1 & II.4; B.II.2) compared with control platelets, thus reflecting the deleterious effect of these GALE variants on the enzymatic activity (Figure 2C, supplemental Figure 3). It is known that proteins involved in apoptotic pathways carry many glycosylation sites, and GALE-deficient human embryonic kidney-293 (HEK-293) cells are hypersensitive to FasL-induced apoptosis.31 To assess the potential effect of hypoglycosylation on platelet lifespan, we evaluated apoptosis-linked caspase activity in platelet lysates. Probands from pedigree A displayed increased levels of both procaspase 8 and cleaved caspase 8, in comparison with control and healthy relatives. In contrast, proband B.II.2 displayed a normal level of procaspase 8 but an increased amount of active caspase 8 (Figure 2D).
Platelets from patients carrying GALE variants show a sharp reduction of GPIbα and glycosylated β1 integrin
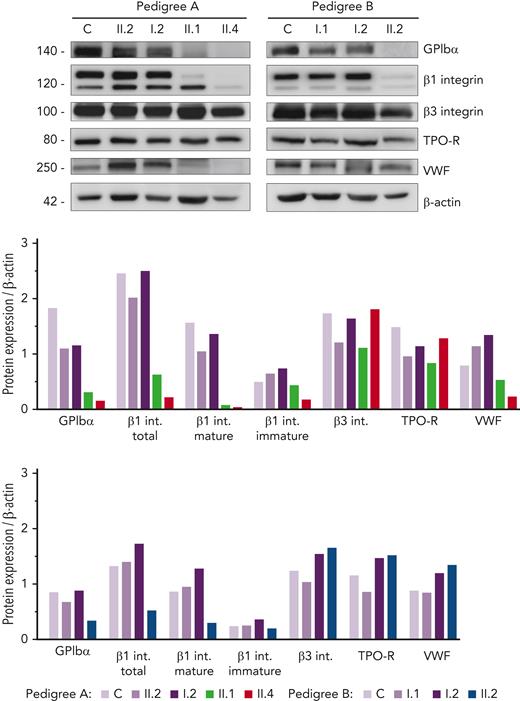
Given the hypoglycosylated profile found in patients’ platelets, we assessed the level of several relevant platelet proteins known to undergo glycosylation. Interestingly, we found a sharp reduction in GPIbα and mature/glycosylated β1 integrin in all probands, whereas the levels of the hypoglycosylated β1 isoform were similar compared with that in control and healthy members (Figure 3). In contrast, normal levels of β3 integrin and TPO receptor (TPO-R) were detected in probands, suggesting that physiological glycosylation and/or maturation of these receptors is preserved in these GALE patients. Assessment of VWF in the platelet lysates showed reduced levels in the probands from pedigree A (Figure 3), in accordance with the high percentage of grey platelets observed in A.II.1 and A.II.4 blood films (54% and 24%, respectively) (Table 1). In contrast, B.II.2 proband, who presented <10% grey platelets in blood film, displayed normal VWF storage (Figure 3; Table 1).
Immunoblotting of platelet lysates revealed severely reduced levels of GPIbα and glycosylated β1 integrin. Platelet lysates of controls, healthy relatives, and patients from both pedigrees were immunoblotted with anti-GPIbα, anti-β1 integrin, anti-β3 integrin, anti-TPO receptor, anti-VWF, and anti-β-actin as an internal control. Band intensities in blots were quantified by densitometric analysis using the ‘Image J’ software. Bar graphs represent each protein expression relative to β-actin. Impaired GPIbα and β1 integrin levels were observed in the proband from both pedigrees. VWF level was also reduced in pedigree A patients. VWF, von Willebrand factor.
Immunoblotting of platelet lysates revealed severely reduced levels of GPIbα and glycosylated β1 integrin. Platelet lysates of controls, healthy relatives, and patients from both pedigrees were immunoblotted with anti-GPIbα, anti-β1 integrin, anti-β3 integrin, anti-TPO receptor, anti-VWF, and anti-β-actin as an internal control. Band intensities in blots were quantified by densitometric analysis using the ‘Image J’ software. Bar graphs represent each protein expression relative to β-actin. Impaired GPIbα and β1 integrin levels were observed in the proband from both pedigrees. VWF level was also reduced in pedigree A patients. VWF, von Willebrand factor.
GALE protein expression increases with MK maturation and is localized in the endoplasmic reticulum (ER)
To get insight of the role of GALE in megakaryopoiesis, we used cord blood–derived CD34+ cells, a well-characterized source of in vitro differentiated human MKs.32 We observed that GALE is poorly expressed in CD34+ hematopoietic stem and progenitor cells (supplemental Figure 4A). During the TPO-induced differentiation, the expression of GALE was increased, being higher in mature MKs (CD61+CD42bhigh) than in immature MKs (CD61+CD42blow) (supplemental Figures 4A-B). In released platelet-like particles, the expression of GALE was reduced (supplemental Figure 4A). These results suggest that GALE is mainly expressed during the late stage of megakaryopoiesis.
To assess the cellular distribution of GALE in MKs, we performed a colocalization assay of GALE with GPIbα (plasma membrane) and the ER protein, calreticulin (supplemental Figure 4C). GALE showed a higher level of colocalization with calreticulin (Pearson’s value, 0.86) than with GPIbα (Pearson’s value, 0.48).
Normal MK maturation but impaired proplatelet formation in patients carrying GALE p.Lys78ValfsX32, p.Thr150Met, p.Val128Met, and p.Leu223Pro variants
To further investigate the mechanisms of thrombocytopenia, we differentiated MKs in vitro from peripheral blood progenitors from probands A.II.4 and B.II.2 and from 5 healthy controls, according to our standard protocols.26,27 We observed normal MK differentiation, evaluated as CD61+ MKs at the end of the culture, between the patients and the healthy controls (Figure 4A). However, we noticed a 60% to 80% reduced expression of GALE in mature MKs from probands A.II.4 and B.II.2 vs healthy controls (Figures 4B-C).
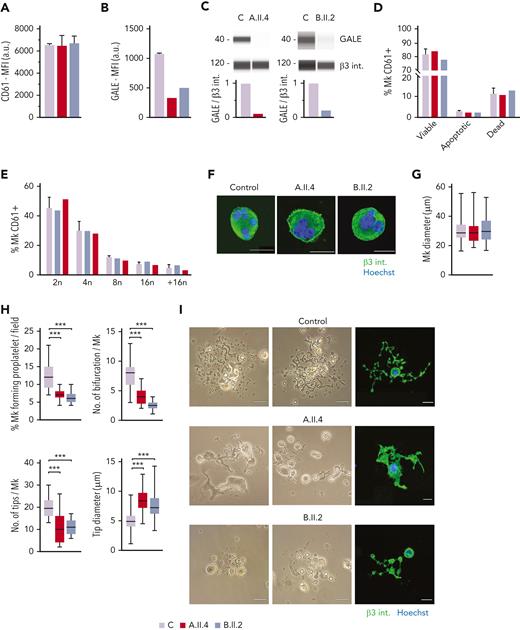
MK culture from probands carrying GALE p.Lys78ValfsX32, p.Thr150Met, p.Val128Met, and p.Leu223Pro variants showed normal polyploidization and maturation but impaired proplatelet formation. MKs were differentiated from peripheral blood progenitor cells from probands A.II.4 and B.II.2 through a 14-days culture, in parallel with healthy controls. (A) MK differentiation assessed as CD61+ cells were measured by MFI by flow cytometry. (B) MFI of GALE, evaluated by flow cytometry, in permeabilized MKs from controls, and probands A.II.4 and B.II.2. Data was relativized with 1 control value. (C) Immunoblotting of MK lysates. Control, A.II.4, and B.II.2 samples were probed with anti-GALE and anti-β3 integrin (internal control). (D) Assessment of MK viability and apoptosis rates at the end of the culture using Annexin V–propidium iodide(PI) labeling. The percentage of CD61+ MKs that are negative for both markers (viable cells), Annexin V+ PI− (apoptotic cells), and double-positive (dead cells) is represented. (E) Percentage of polyploid MKs after PI labeling. (F) Representative images of mature and polynuclear MKs labeled with an anti–β3 integrin antibody (green fluorescence). Hoechst (blue) was used for counterstaining nuclei. Scale bars, 20 μm. (G) Diameter measurement of MK from A.II.A, B.II.2, and healthy controls, in fibrinogen-coated coverslips (n = 100). (H) Characterization of impaired proplatelet formation in fibrinogen-coated coverslips in probands A.II4 and B.II.2 vs control, (1) Rate of proplatelet formation measured as the proportion of MKs displaying ≥1 proplatelet with respect to the total number of MKs; (2) Number of bifurcations of proplatelet shafts per MK; (3) Number of proplatelet-free ends (tips); (4) Diameter measurement of proplatelet-free ends (tips) from A.II.4, B.II.2, and healthy control (n = 100). Box and whiskers graphs are shown. The central line represents the median value, whereas percentiles 25 to75 is included in the box. Whiskers represent the minimum and maximum values. (I) Representative image of proplatelet formation in optical and immunofluorescence microscope. MKs was plated on fibrinogen-coated coverslips and incubated for 16 hours at 37°C and 5% CO2. Cells were stained with an anti-CD61 antibody (green). Hoechst (blue) was used for counterstaining nuclei. Scale bars, 20 μm. ∗∗∗P < .001; 2-tailed Student t test.
MK culture from probands carrying GALE p.Lys78ValfsX32, p.Thr150Met, p.Val128Met, and p.Leu223Pro variants showed normal polyploidization and maturation but impaired proplatelet formation. MKs were differentiated from peripheral blood progenitor cells from probands A.II.4 and B.II.2 through a 14-days culture, in parallel with healthy controls. (A) MK differentiation assessed as CD61+ cells were measured by MFI by flow cytometry. (B) MFI of GALE, evaluated by flow cytometry, in permeabilized MKs from controls, and probands A.II.4 and B.II.2. Data was relativized with 1 control value. (C) Immunoblotting of MK lysates. Control, A.II.4, and B.II.2 samples were probed with anti-GALE and anti-β3 integrin (internal control). (D) Assessment of MK viability and apoptosis rates at the end of the culture using Annexin V–propidium iodide(PI) labeling. The percentage of CD61+ MKs that are negative for both markers (viable cells), Annexin V+ PI− (apoptotic cells), and double-positive (dead cells) is represented. (E) Percentage of polyploid MKs after PI labeling. (F) Representative images of mature and polynuclear MKs labeled with an anti–β3 integrin antibody (green fluorescence). Hoechst (blue) was used for counterstaining nuclei. Scale bars, 20 μm. (G) Diameter measurement of MK from A.II.A, B.II.2, and healthy controls, in fibrinogen-coated coverslips (n = 100). (H) Characterization of impaired proplatelet formation in fibrinogen-coated coverslips in probands A.II4 and B.II.2 vs control, (1) Rate of proplatelet formation measured as the proportion of MKs displaying ≥1 proplatelet with respect to the total number of MKs; (2) Number of bifurcations of proplatelet shafts per MK; (3) Number of proplatelet-free ends (tips); (4) Diameter measurement of proplatelet-free ends (tips) from A.II.4, B.II.2, and healthy control (n = 100). Box and whiskers graphs are shown. The central line represents the median value, whereas percentiles 25 to75 is included in the box. Whiskers represent the minimum and maximum values. (I) Representative image of proplatelet formation in optical and immunofluorescence microscope. MKs was plated on fibrinogen-coated coverslips and incubated for 16 hours at 37°C and 5% CO2. Cells were stained with an anti-CD61 antibody (green). Hoechst (blue) was used for counterstaining nuclei. Scale bars, 20 μm. ∗∗∗P < .001; 2-tailed Student t test.
No differences in MK viability was found between controls and the probands A.II.4 and B.II.2 (Figure 4D). Indeed, both patients’ MKs displayed a similar percentage of polyploid cells with respect to the healthy controls (Figure 4E) and normal MKs size and maturation (Figures 4F-G). However, both patients A.II.4 and B.II.2 had a significantly reduced proportion of MKs extending proplatelets vs controls, both in suspension (data not shown) and in adhesion on fibrinogen (A.II.4, 7.3%; and B.II.2, 6.2% vs Controls, 12.3%) (Figures 4H-I). The patient’s proplatelets exhibited an impaired morphology that was characterized by reduced length and bifurcation of the shafts, resulting in a smaller number of proplatelet-free ends and tips with increased size (Figures 4H-I).
GALE p.Lys78ValfsX32, p.Thr150Met, p.Val128Met, and p.Leu223Pro affect GPIbα and β1 integrin glycosylation, externalization, and delivery to platelets
Probands and the healthy controls had a comparable surface expression of the β3 integrin but reduced GPIbα (A.II.4, 22%; B.II.2, 38% vs the healthy control, 89%) and β1 integrin (A.II.4, 58%; B.II.2, 33% vs the healthy control, 114%) (Figures 5A). The total levels of GPIbα and β1 integrin in both patients were similar to that in control MKs (Figure 5A), thus suggesting that the observed reduction at the MK and platelet surfaces could be because of their impaired externalization (Figures 3 and 5A). Immunoblotting analysis of MK lysates revealed comparable levels of GPIbα and β1 integrin between probands and healthy controls but the presence of bands of a slightly lower molecular weight, probably because of the hypoglycosylation profile of these proteins (Figure 5B). To further confirm their reduced exposure to the membrane and a preferential accumulation of the hypoglycosylated forms within the ER, we assessed their colocalization with the ER protein, calreticulin. We found that GPIbα and β1 integrin were increasingly associated with calreticulin in probands A.II.A and B.II.2 vs the healthy control (GPIbα, 61% and 66% vs 28%, respectively; β1 integrin, 83% and 89% vs 51%, respectively) (Figure 5C). Moreover, GPIbα and β1 integrin fluorescence was distributed at the plasma membrane of control Mks, whereas the signal was mainly localized within the cytoplasm of patients’ MKs (Figure 5D).
MKs from probands carrying GALE variants displayed impaired GPIbα and β1 glycosylation and externalization. MKs were differentiated from peripheral blood progenitor cells (probands A.II.4 and B.II.2) through a 14-days culture, in parallel with healthy controls. (A) MFI of β3 integrin (anti-CD61), GPIbα (anti-CD42b), and β1 integrin (anti-CD29) in permeabilized MKs (total levels) and nonpermeabilized MKs (surface levels) from controls, A.II.4, and B.II.2 are represented. Data were normalized against 1 control value. (B) Representative analysis of the automized immunoblotting of GPIbα and Western blotting of β1 integrin from a healthy control and proband B.II.2. Red dotted lines highlight bands of lower molecular weight in MKs from patients. Densitometry analysis demonstrated comparable levels of total proteins between patients and controls. β3 integrin was used as an internal control. (C) Representative image of control, A.II.4, and B.II.2 MKs. Cells were cytospinned and stained with anti-GPIbα or anti-β1–integrin antibodies (green). The ER was stained with anti-calreticulin (red fluorescence). Hoechst (blue) was used for counterstaining nuclei. Pearson’s R values indicate the colocalization rate between GPIbα or β1 integrin with calreticulin in control and probands A.II.4 and B.II.2 MKs. (D) Fluorescence intensity distribution of GPIbα and β1 integrin in control vs patients. Control MKs presented a major distribution of GPIbα and β1 integrin in the membrane, with reduced intracellular levels, whereas patients’ fluorescence distribution was mainly intracellular. Scale bars, 10 μm. ∗∗∗P < .001; ∗∗P < .01; 2-tailed Student t test. CALR, calreticulin.
MKs from probands carrying GALE variants displayed impaired GPIbα and β1 glycosylation and externalization. MKs were differentiated from peripheral blood progenitor cells (probands A.II.4 and B.II.2) through a 14-days culture, in parallel with healthy controls. (A) MFI of β3 integrin (anti-CD61), GPIbα (anti-CD42b), and β1 integrin (anti-CD29) in permeabilized MKs (total levels) and nonpermeabilized MKs (surface levels) from controls, A.II.4, and B.II.2 are represented. Data were normalized against 1 control value. (B) Representative analysis of the automized immunoblotting of GPIbα and Western blotting of β1 integrin from a healthy control and proband B.II.2. Red dotted lines highlight bands of lower molecular weight in MKs from patients. Densitometry analysis demonstrated comparable levels of total proteins between patients and controls. β3 integrin was used as an internal control. (C) Representative image of control, A.II.4, and B.II.2 MKs. Cells were cytospinned and stained with anti-GPIbα or anti-β1–integrin antibodies (green). The ER was stained with anti-calreticulin (red fluorescence). Hoechst (blue) was used for counterstaining nuclei. Pearson’s R values indicate the colocalization rate between GPIbα or β1 integrin with calreticulin in control and probands A.II.4 and B.II.2 MKs. (D) Fluorescence intensity distribution of GPIbα and β1 integrin in control vs patients. Control MKs presented a major distribution of GPIbα and β1 integrin in the membrane, with reduced intracellular levels, whereas patients’ fluorescence distribution was mainly intracellular. Scale bars, 10 μm. ∗∗∗P < .001; ∗∗P < .01; 2-tailed Student t test. CALR, calreticulin.
B.II.2 patient platelets displayed severely reduced adhesion onto VWF, moderate decrease onto laminin and fibronectin, and slight impaired adhesion onto collagen, compared with control platelets (supplemental Figure 5). No results are available for patient A.II.4. However, the lack of GPIbα and β1 integrin in the MK surface, makes it conceivable that these receptors cannot be delivered to mature platelets, thus affecting their adhesion to physiologically relevant extracellular matrices.
GALE variants altered the distribution of filamin A and actin in MKs from patients
Because filamin A promotes cellular adhesion by linking membrane glycoproteins such as GPIbα to the actin cytoskeleton,33 we investigated several structural proteins in MKs from both patients with GALE variants. As shown in Figure 6A, control MKs displayed a uniform actin distribution, colocalizing with filamin A, in mature MKs, in the proplatelets, and in the proplatelet tips. However, in MKs from probands A.II.4 and B.II.2, we observed a heterogeneously disorganized and nonuniform actin cytoskeleton, actin filaments distributed in patches, and a heterogeneous delocalization between filamin A and actin, forming actin clusters with decreased levels of filamin A (Figure 6A). In contrast, no differences were found in microtubule assembly (Figure 6B).
GALE p.Lys78ValfsX32, p.Thr150Met, p.Val128Met, and p.Leu223Pro variants impaired actin and filamin A distribution among the MK cytoplasm. MKs were differentiated from peripheral blood progenitor cells (probands A.II.4 and B.II.2) through a 14-days culture, in parallel with healthy controls. (A) Representative image of MKs stained with phalloidin (green) and anti-filamin A (red) antibodies in control, A.II.4, and B.II.2 samples. We observed a heterogeneously disorganized and nonuniform actin cytoskeleton, based on the presence of actin filaments distributed in patches (white asterisk), a heterogeneous delocalization of filamin A with actin, and the presence of actin in clusters with reduced levels of filamin A (white arrow). (B) Representative image of MKs stained with an anti-β1–tubulin to investigate microtubule assembly in MKs forming proplatelets. Hoechst (blue) was used for counterstaining nuclei. Scale bars, 20 μm. (C) Immunoblotting of Mk lysates. Control, A.II.4, and B.II.2 samples were probed with anti-β actin and anti-β3 integrin (internal control).
GALE p.Lys78ValfsX32, p.Thr150Met, p.Val128Met, and p.Leu223Pro variants impaired actin and filamin A distribution among the MK cytoplasm. MKs were differentiated from peripheral blood progenitor cells (probands A.II.4 and B.II.2) through a 14-days culture, in parallel with healthy controls. (A) Representative image of MKs stained with phalloidin (green) and anti-filamin A (red) antibodies in control, A.II.4, and B.II.2 samples. We observed a heterogeneously disorganized and nonuniform actin cytoskeleton, based on the presence of actin filaments distributed in patches (white asterisk), a heterogeneous delocalization of filamin A with actin, and the presence of actin in clusters with reduced levels of filamin A (white arrow). (B) Representative image of MKs stained with an anti-β1–tubulin to investigate microtubule assembly in MKs forming proplatelets. Hoechst (blue) was used for counterstaining nuclei. Scale bars, 20 μm. (C) Immunoblotting of Mk lysates. Control, A.II.4, and B.II.2 samples were probed with anti-β actin and anti-β3 integrin (internal control).
Finally, we observed similar levels of β-actin in both probands’ MKs compared with controls’, suggesting a qualitative but not quantitative defect in the MK cytoskeletal proteins in patients with GALE variants (Figure 6C).
Patients with GALE variants displayed VWF delocalization within the MK membrane
We lastly examined the VWF distribution, and we observed that mature and proplatelet-forming MKs from controls had the signal from granules both within the cytoplasm and near the cell membrane, which is an essential step to ensure physiological proplatelet formation (Figure 7A-B).28 Nevertheless, A.II.4 and B.II.2 MKs displayed a preferential location of VWF within the cell cytoplasm, with a significant reduction in its localization at the plasma membrane (Figure 7A-B). This alteration was more pronounced in proband A.II.4 (Figure 7B) and could justify the presence of grey platelets in peripheral blood (Figures 1B and 3).
Carriers of GALE p.Lys78ValfsX32, p.Thr150Met, p.Val128Met, and p.Leu223Pro variant displayed VWF delocalization within the MK membrane. MKs were differentiated from peripheral blood progenitor cells (probands A.II.4 and B.II.2) through a 14-days culture, in parallel with healthy controls. (A) Representative image of alpha-granules in mature-polyploid MKs and MK forming proplatelets in control, A.II.4, and B.II.2. Cells were stained with an anti-β3 integrin antibody (green). Alpha-granules were stained with anti-VWF (red). Hoechst (blue) was used for counterstaining nuclei. Arrows indicate the presence of VWF in the control MK membrane, whereas both A.II.4 and B.II.2 had a severely reduced expression of VWF in the membrane. (B) Fluorescence intensity distribution of VWF, relativized with β3 integrin (ratio VWF-β3 integrin), in the MK membrane from healthy controls, A.II.4, and B.II.2. Scale bars, 20 μm. ∗∗∗P < .001; ∗P < .05; 2-tailed Student t test.
Carriers of GALE p.Lys78ValfsX32, p.Thr150Met, p.Val128Met, and p.Leu223Pro variant displayed VWF delocalization within the MK membrane. MKs were differentiated from peripheral blood progenitor cells (probands A.II.4 and B.II.2) through a 14-days culture, in parallel with healthy controls. (A) Representative image of alpha-granules in mature-polyploid MKs and MK forming proplatelets in control, A.II.4, and B.II.2. Cells were stained with an anti-β3 integrin antibody (green). Alpha-granules were stained with anti-VWF (red). Hoechst (blue) was used for counterstaining nuclei. Arrows indicate the presence of VWF in the control MK membrane, whereas both A.II.4 and B.II.2 had a severely reduced expression of VWF in the membrane. (B) Fluorescence intensity distribution of VWF, relativized with β3 integrin (ratio VWF-β3 integrin), in the MK membrane from healthy controls, A.II.4, and B.II.2. Scale bars, 20 μm. ∗∗∗P < .001; ∗P < .05; 2-tailed Student t test.
Discussion
In the last decade, high-throughput sequencing technology and particularly WES, has revolutionized the genetic landscape of ITs with the identification of novel causative genes.34-36 One of these is the GALE gene,37 encoding an enzyme, the UDP-galactose-4-epimerase, which is involved in a wide range of biological processes like N-linked and O-linked glycosylation (supplemental Figure 3).31 Genetic variantions in GALE cause an autosomal recessive disorder (Online Mendelian Inheritance in Man, OMIM database 230350). Compound heterozygous variants of GALE usually provoke partial impairment of GALE activity, being associated with nongeneralized forms or even asymptomatic presentations, whereas homozygous variants are related to severe forms of this condition, which usually present generalized galactosemia.38-41 In addition, long-term complications may include learning difficulties, developmental delay, and poor growth,42 although cardiac failure and dysmorphic features are less common manifestations.43 Until recently, there was no evidence linking GALE defects and hematological alterations. In 2019, Seo et al reported for the first time 6 patients from 1 pedigree affected by severe thrombocytopenia, febrile neutropenia, and mild anemia, who were homozygous for the GALE p.Arg51Trp variant.19 Febres-Aldana et al have recently described a child with bone marrow dysfunction and complex congenital heart disease associated with compound heterozygosity in GALE (p.Arg51Trp and p.Gly237Asp).20 In 2021, Markovitz et al reported a patient with pancytopenia and immune dysregulation because of a previously reported homozygous GALE variant (p.Thr150Met).21 In these previous reports, the authors agreed that the identification of new patients and/or further in vitro studies were necessary to consolidate the correlation between GALE mutations and abnormal organ morphogenesis or hematologic disorders. In contrast with previously reported cases, our patients with compound heterozygosity of 4 variants affecting GALE displayed a syndromic type of IT, characterized by mental retardation, cardiovascular abnormalities, jaundice, giant and/or grey platelets, and remarkable reduction in granule secretion.
From a mechanistic point of view, these variants caused a severe reduction in the protein levels and the enzymatic activity of UDP-galactose-4-epimerase, which is reflected in a strongly impaired N-linked and O-linked protein glycosylation in platelets11,14,44 In addition, the fact that all the phases of the heart valve morphogenesis are mediated by N-glycosylated proteins may explain that our 3 probands had mitral valve prolapse that had to be corrected at birth.20,42,43 Taken together, these data pointed out the essential role of GALE in the glycosylation process. Proper performance of glycosylation is critical for physiological hematopoiesis because it would favor proliferation and clearance of the hematopoietic precursors,11,44 which are especially relevant in platelet development.44,45 The hypoglycosylation profile found in platelets from our patients was associated with reduction in surface expression of the GPIb-IX-V complex and almost absence of glycosylated β1 integrin. Also, hypoglycosylated platelets were prone to increased apoptosis, in accordance with previous studies.46,47
Recently, thrombocytopenia has been described in other congenital disorders of glycosylation/sialylation, including the GNE-related disorder, but the mechanisms remain unclear.15,16,48,49 To the best of our knowledge, there is scarce information about the performance of megakaryopoiesis in patients with thrombocytopenia associated with GALE variants. Here, we showed that GALE variants disrupt thrombopoiesis because of the impaired glycosylation of the GPIbα and the β1 integrin and altered distribution of filamin A and F-actin cytoskeleton, leading to a reduction in proplatelet formation (supplemental Figure 6).
It is well established that polyploidization is dispensable for terminal MK maturation.50 Normal ploidy was observed in MKs cultured from probands A.II.4 and B.II.2. Mature MK develop the demarcation membrane system (DMS), suffering a remodeling into pseudopodia by a process based on microtubules, especially the β1 tubulin, which guides proplatelet shaft initiation and elongation23,51,52 and the actomyosin cytoskeleton that is critical for the DMS formation,53,54 and to drive the formation of proplatelets.55
There is a great controversy not only about the events that give rise to DMS and the subsequent formation of platelets during thrombopoiesis, but also about the role of GPIbα during the DMS and proplatelet formation. The absence of the GPIb-IX-V complex on the MK surface is associated with impaired proplatelet formation, as described in Bernard Soulier syndrome.56 MKs from GPIbα-null mice have a poorly developed DMS and a reduced internal membrane pool,57 whereas the GPIbβ-null mice displayed abnormal DMS formation, and the microtubule bundles at the core of the proplatelet and within the marginal band are measurably thicker than those in wild type platelets, suggesting that GPIbβ could regulate microtubule organization.58 Indeed, the analysis of the ultrastructure of a patient with Bernard Soulier syndrome uncovered altered DMS, disorganized microtubules, and platelets with sparse or absent granulation.59,60
Both probands A.II.4 and B.II.2 showed an unaffected microtubule assembly despite their impaired ability to extend proplatelets, reinforcing the hypothesis that proplatelet formation is contributed by multiple elements. Indeed, impaired filamin A and actin distribution, among the MK cytoskeleton, was observed, which may be expected because GPIbα is not expressed on the MK surface, disrupting the binding to filamin A. It has been previously described in FLNA-RD the distribution of both filamin A and actin in patches, as observed here in our patients, MKs containing a large number of granules with a heterogeneous distribution, and a well-developed DMS concentrated in discrete domains.61 There is increasing evidence that GPIbα–filamin A interaction regulates platelet size. Indeed, the abnormal architecture of giant platelets associated with GPIbα alterations has been proposed to arise from the absence of GPIbα–filamin A interaction.33,62 It has also been described that the synthesis of mature GPIbα in CHO-GPIbα/β/IX cells is eliminated by brefeldin A, which inhibits the exit from the ER, and it is restored by lactacystin, proving that GPIbα binds to filamin A within the ER and that filamin A binding directs post-ER trafficking of GPIbα to the cell surface.63 This further supports the evidence that the impaired distribution of filamin A is a consequence of the reduced glycosylation and externalization of the GPIbα in our patients with GALE.
Moreover, impaired megakaryopoiesis has been shown in a patient with thrombocytopenia who carried a mutation in the GPIbα interaction site with VWF, and blockade of the extracellular portion of GPIbα was shown to inhibit MK differentiation and proplatelet formation in CD34+ cells.64,65 It is well known that the interaction between filamin A and GPIbα positively modulates VWF receptor function.62 The VWF relocation on the MK plasma membrane and binding to GPIbα is crucial for proplatelet formation.28 In both patients, we observed a reduced expression of VWF on the MK membrane. This alteration is more remarkable in proband A.II.4, whose clinical symptoms are more serious, with even lower levels of GALE, lower platelet counts, decreased GPIb-IX expression in platelets, and decreased granule secretion than B.II.2. Occurance of this severe phenotype could be justified by the fact that patient A.II.4 has a stop codon in GALE (p.Lys78ValfsX32 variant).
Reduced levels of GALE in MKs are not only associated with the hypoglycosylation of GPIbα, but also of the β1 integrin. Both are retained in the ER and not delivered to the surface of mature platelets. β1 integrin signaling, which is required for regulating MK function and proplatelet formation,66,67 was shown to be dependent on LacNac, which is regulated by β-1,4-galactosyltransferase. Alterations at this level has also been associated with thrombocytopenia.14,44
In contrast, the expression and/or function of other receptors of the megakaryocytic lineage, such as β3 integrin and TPO receptor, were preserved, thus suggesting that different pathways of glycosylation are likely to be responsible for specific targets. Extending the analysis to additional receptors, such as the transferrin receptor or other crucial proteins whose function depends on glycosylation, may open new paths toward the understanding of the impact of glycosylation in megakaryopoiesis. Importantly, the presence of a fully functional TPO receptor in mutant MKs raises a promising horizon for the treatment of patients with GALE with TPO analogues to rescue the severe thrombocytopenia, as an alternative to transfusions.26 Besides, gene therapy may be a promising approach for the future of these patients.
In summary, we have identified 4 variants in GALE that cause syndromic thrombocytopenia. These variants severely reduced the enzymatic activity of UDP-galactose-4-epimerase resulting in impaired platelet glycosylation. In vitro differentiated MKs from patients carrying these GALE variants showed reduced externalization of GPIbα and β1 integrin into the cell membrane, likely leading to the impaired proplatelet formation because of the altered distribution of filamin A and actin in MKs. Our new data strongly reinforce the role of GALE in glycosylation and unveil novel mechanisms underlying the GALE-related thrombocytopenia mediated by defective glycosylation of β1 integrin and the GPIb-IX-V complex.
Acknowledgments
The authors thank the patients and family members for their kind collaboration during the study. Eva Lumbreras, Silvia Tocino Antonio, Sara González Briones, Irene Rodríguez Iglesias and Daisy Castiñeiras-Ramos for technical support with experiments; the CIC-IBMCC Microscopy and Cytometry Service for technical assistance with the confocal immunofluorescence studies; María de los Ángeles Manrique Gonzalo, Nuria Vicente Holgado, Mercedes Rodríguez Martín, Isabel Ramos Sevillano, María del Mar Cambronero Estévez and Beatriz Oreja Martín for blood samples collection, hemograms, and blood films; ‘Centro Grandi Strumenti’ of the University of Pavia, Italy, for technical assistance with flow cytometry and confocal microscopy; Cesare Perotti and Claudia Del Fante of the I.R.C.C.S. Policlinico San Matteo of Pavia, Italy, for providing cord blood samples; the group of Carlo Gaetano of the I.R.C.C.S. Fondazione Maugeri of Pavia, Italy, for helping with WES and ProteinSimple analysis. The visual abstract was created using Biorender.com.
This work was supported by grants from Instituto de Salud Carlos III (ISCIII) & Feder (PI17/01966, PI20/00926) and cofunded by European Union (ERDF/ESF, “Investing in your future”), Gerencia Regional de Salud (GRS2061/A/2019, GRS2135/A/2020, GRS2314/A/2021), Fundación Mutua Madrileña (FMM, AP172142019), Sociedad Española de Trombosis y Hemostasia (SETH-FETH; Premio López Borrasca 2019 and Ayuda a Grupos de Trabajo en Patología Hemorrágica 2020 and 2021), Fundación Castellano Leonesa de Hematología y Hemoterapia (FUCALHH 2020), Red Temática de Investigación Cooperativa en Cáncer (RTICC) (RD12/0036/0069), Centro de Investigación Biomédica en Red de Cáncer (CIBERONC CB16/12/00233). Progetti di ricerca di rilevante interesse Nazionale (PRIN 2017Z5LR5Z), and the European Commission (H2020-FETOPEN-1-2016-2017-SilkFusion ID 767309). The author´s research on Inherited Platelet Disorders is conducted in accordance with the aims of the multicentric project “Functional and Molecular Characterization of Patients with Inherited Platelet Disorders” of Grupo Español de Alteraciones Plaquetarias Congénitas (GEAPC). A.M.-Q. is fully supported by an “Ayuda predoctoral de la Junta de Castilla y León” by the Fondo Social Europeo (JCYL- EDU/556/2019 PhD scholarship) and received an “Ayuda para breves estancias formativas” from the Sociedad Española de Hematología y Hemoterapia (SEHH-FEHH), and from the Sociedad Española de Trombosis y Hemostasia (SETH-FETH); E.V. is fully supported by an “Ayuda para contratos predoctorales de la Universidad de Salamanca cofinanciadas por el banco Santander,” programa propio III convocatoria 2018; I.S.-G. is supported by a contract from the University of Salamanca cofinanced by the Junta de Castilla y León (Council of Education) and FEDER-European Union [ref. SA0118P20 (2)]; S.S.-M. and C.M.-G. received funding from the European Research Council (ERC) under the ERA-Per-Med programme (ERAPERMED2018-275) SYNtherapy and ISCIII (AC18/00093) cofunded by ERDF/ESF, “Investing in your future”; I.G.-T. and R.B. are supported by a grant from the Universidad de Salamanca (“Contrato postdoctoral Universidad de Salamanca programa propio II, 2019”)
Authorship
Contribution: J.M.B., I.G.-T., and A.B. designed the research; A.M.-Q., C.A.D.B., E.V., L.D.-A., J.R., and I.S.-G. performed the functional experiments; C.A.D.B., V.A., and A.M.-Q. performed glycosylation assays; A.M.-Q., C.A.D.B., and P.M.S. performed human megakaryocyte cultures; S.S.-M. and C.M.-G. prepared samples for whole-exome sequencing; A.M.-Q., S.S.-M., C.M.-G., R.B., J.M.H.-R., and J.M.B. conducted whole–exome sequencing analysis and interpretation; P.R.-S. performed enzymatic analysis and interpretation; M.J.P., E.P., and J.R.G.-P. provided clinical information and managed the patients; A.M.-Q., J.R., C.A.D.B., I.G.-T., A.B. and, J.M.B. analyzed and interpreted the results; A.M.-Q. and C.A.D.B. prepared figures and illustrations; A.M.-Q., C.A.D.B., J.R., A.B. and J.M.B. wrote the paper; and all authors reviewed the results and approved the final version of the manuscript.
Conflict-of-interest disclosure: M.J.P. reports honoraria from Abbvie, Celgene, Janssen, Roche, Servier, Takeda., Astra-Zeneca and Gilead, and participation on Advisory Board from Amgen, Janssen, Roche, Takeda and Abbvie outside of the submitted work; J.M.H.-R. reports research support from Novartis and Celgene/BMS, consulting fees from GSK, honoraria from Amgen, Novartis, Celgene/BMS, Pfizer, GSK, and participation on Advisory Board from Novartis, Pfizer, Amgen and Celgene/BMS outside of the submitted work; J.R.G.-P. reports honoraria from Novo Nordisk, Shire, SOBI, Roche, Daiichi Sankyo, Pfizer, Rovi, Amgen, and Novartis and participation on Advisory Board from Amgen, Novartis, SOBI, Grifols and CSL Behring outside of the submitted work; J.R. reports honoraria from NovoNordisk and participation on Advisory Board from Terumo BCT outside of the submitted work; J.M.B. reports honoraria from NovoNordisk, Roche, Takeda, CSL Behring, Sobi, Novartis, Janssen, and Rovi and participation on Advisory Board from Sobi, Novartis outside of the submitted work. The remaining authors declare no competing financial interests.
Correspondence: José María Bastida, Unidad de Trombosis y Hemostasia, Complejo Asistencial Universitario de Salamanca (CAUSA), 37007 Salamanca, Spain; e-mail: jmbastida@saludcastillayleon.es; Alessandra Balduini, Dipartimento di Medicina Molecolare, Università degli Studi di Pavia, 27100 Pavia, Italy; e-mail: alessandra.balduini@unipv.it; and Ignacio García-Tuñón, Departamento de Medicina, Universidad de Salamanca, 37007 Salamanca, Spain; e-mail: ignacio.tunon@uah.es.
References
Author notes
High-throughput sequencing data are available at Sequence Read Archive database under accession number PRJNA839436.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
A.M.-Q. and C.A.D.B. contributed equally to this study.
J.M.B., A.B., and J.R. are joint senior authors.
On behalf of “Grupo Español de Alteraciones Plaquetarias Congénitas (GEAPC)”, Sociedad Española de Trombosis y Hemostasia (SETH).




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal