In this issue of Blood, Hulbert and colleagues demonstrate the normalization of cerebral hemodynamics following hematopoietic stem cell transplantation (HSCT) in 10 children with sickle cell disease (SCD).1 To place their observations into context, they compared cerebral blood flow (CBF) and regional brain oxygen extraction fraction (rOEF) in SCD patients prior to and following HSCT, prior to and following transfusion, and in normal children.
To compensate for chronic anemia, the brain increases CBF inversely with hemoglobin level to preserve whole brain oxygen delivery.2 Increased resting CBF leaves the brain with little vasodilatory reserve and vulnerable to intermittent disruption of oxygen delivery under stress.3 More importantly, compensatory CBF increases favor the cerebral cortex at the expense of deep white matter structures.4,5 Previous work by the authors has clearly demonstrated increased rOEF in watershed areas lying between vascular territories.6 Regional OEF increased proportionally to anemia severity and exhibited high spatial concordance to regions of high silent stroke risk.6
Successful HSCT restores oxygen-carrying capacity to normal levels by increasing hemoglobin levels and eliminating or markedly attenuating the sickling process. A priori, one would expect relaxation of the brain’s compensatory cerebral hemodynamic changes, but complete normalization has not previously been demonstrated. Long-term transfusion therapy produced qualitatively similar CBF and rOEF changes, but the effect sizes were significantly smaller. One of the more notable findings of the article was that CBF and rOEF values were independent of treatment (HSCT vs transfusion, pre- vs posttreatment), after controlling for hemoglobin. This suggests that the primary advantage of HSCT over long-term transfusion therapy was the restoration of normal hemoglobin levels.
This observation is important because long-term transfusion therapy in SCD is performed to lower hemoglobin S to below a target level (typically 30%), not to restore oxygen-carrying capacity. With automated erythrocytapheresis, it is possible to lower hemoglobin S percentage with no change in total hemoglobin, although most centers allow for some increase in posttransfusion hemoglobin. Although using a hemoglobin S target has merit for lowering overt stroke rate, it offers incomplete protection for silent stroke.7 Thus, the present work by Hulbert et al suggests a mechanism for continued white matter injury in SCD patients undergoing long-term transfusion.
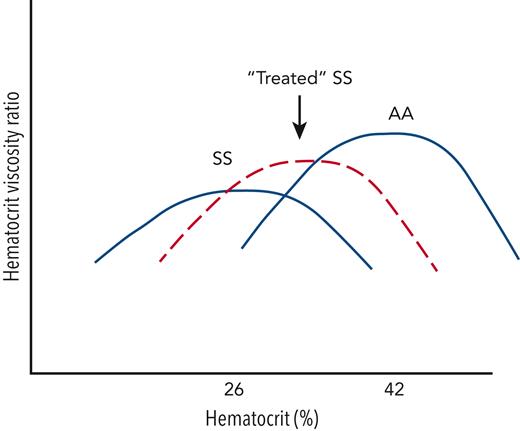
The optimal oxygen-delivery potential in the microvasculature is best approximated by the ratio of hematocrit to viscosity. This ratio balances the benefits of oxygen-carrying capacity (which rises linearly with hematocrit) and the penalties from viscosity (which rises nonlinearly with hematocrit). The figure demonstrates putative hematocrit-viscosity ratio (HVR) curves for patients having SS hemoglobin and AA hemoglobin. Given that SS blood is more viscous for any given hematocrit than AA blood, the hematocrit that optimizes microvascular oxygen delivery is lower in SS patients.8 The viscosity penalty is worse in SS than many other forms of anemia, so it is not surprising that rOEF is worse in SCD than non-sickle anemias for any given hematocrit value.9 Effective SCD therapies, such as hydroxyurea and blood transfusions, act not only to improve hematocrit but to improve blood viscosity as well.
Schematic illustrating the HVR as a function of hematocrit for patients with SS hemoglobin (untreated), SS hemoglobin (treated with hydroxyurea or chronic transfusions), and AA hemoglobin. HVR is a metric of the microvascular oxygen-delivery potential and balances the positive effects of increased oxygen delivery vs the negative effects of increased viscosity. The hematocrit that optimizes HVR, as well as the peak HVR obtained, is lower in patients with SS compared with those with AA hemoglobin. In SCD, effective therapies must not only increase hemoglobin but improve red cell rheology.
Schematic illustrating the HVR as a function of hematocrit for patients with SS hemoglobin (untreated), SS hemoglobin (treated with hydroxyurea or chronic transfusions), and AA hemoglobin. HVR is a metric of the microvascular oxygen-delivery potential and balances the positive effects of increased oxygen delivery vs the negative effects of increased viscosity. The hematocrit that optimizes HVR, as well as the peak HVR obtained, is lower in patients with SS compared with those with AA hemoglobin. In SCD, effective therapies must not only increase hemoglobin but improve red cell rheology.
Given this physiology, should the goals for posttransfusion hemoglobin levels be raised in SCD patients undergoing long-term transfusion to improve oxygen delivery to deep white matter structures in addition to suppressing hemoglobin S? In the present study, the hemoglobin level in sickle cell patients undergoing long-term transfusion cycled between a median of 9.1 g/dL pretransfusion and 10.6 g/dL posttransfusion. This regimen ameliorated CBF and rOEF but still leaves the deep white matter ischemic compared with control patients. Long-term transfusion increases blood viscosity in SCD patients, improving HVR at high shear but not low shear.10 In contrast, HSCT resulted in a median hemoglobin level of 12.4 g/dL and complete restoration of normal blood viscosity, leading to normal oxygen-delivery potential. Although this study does not compare HSCT with long-term transfusion with more aggressive transfusion thresholds, it certainly suggests that our current transfusion practices expose the brain to intermittent, detrimental anemia and cerebral hypoxia.
The present work also provides important context into other potential therapies in SCD undergoing clinical trials. The present data suggest that cerebral risk exhibits a graded response with hemoglobin level. For the average patient in this study, a 1 g increase in hemoglobin would yield >10% improvement in oxygen-carrying capacity. As the number of approved therapies increases, combination therapies may allow hemoglobin levels closer to the lower limits of normal. This work also suggests that current concepts regarding the acceptability of moderate anemia (hemoglobin levels <10 g/dL) need to be reconsidered in the context of cerebral oxygen delivery and brain damage.
Conflict-of-interest disclosure: J.C.W. is a paid member of the National Heart, Lung, and Blood Institute Sickle Cell Disease Advisory Committee and has grant funding from Philips Healthcare. He also serves as a consultant for Agios, Celgene, Imago Biosciences, Pharmacosmos, Regeron, Silence Therapeutics, Vifor, and World Care Clinical.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal