Key Points
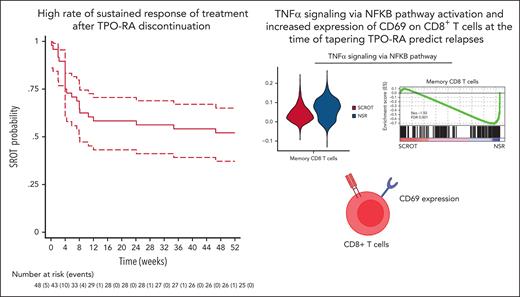
High rate of sustained response after TPO-RA discontinuation in patients with persistent or chronic ITP achieving initial CR on treatment.
Increased expression of CD69 on CD8+ T cells at the time of tapering TPO-RA could predict relapses.
Abstract
Sustained response off treatment (SROT) after thrombopoietin receptor agonist (TPO-RA) discontinuation has been reported in immune thrombocytopenia (ITP). This prospective multicenter interventional study enrolled adults with persistent or chronic primary ITP and complete response (CR) on TPO-RAs. The primary end point was the proportion of patients achieving SROT (platelet count >30 × 109/L and no bleeding) at week 24 (W24) with no other ITP-specific medications. Secondary end points included the proportion of sustained CR off-treatment (SCROT, platelet count >100 × 109/L and no bleeding) and SROT at W52, bleeding events, and pattern of response to a new course of TPO-RAs. We included 48 patients with a median age of 58.5 years; 30 of 48 had chronic ITP at TPO-RA initiation. In the intention-to-treat analysis, 27 of 48 achieved SROT, 15 of 48 achieved SCROT at W24; 25 of 48 achieved SROT, and 14 of 48 achieved SCROT at W52. No severe bleeding episode occurred in patients who relapsed. Among patients rechallenged with TPO-RA, 11 of 12 achieved CR. We found no significant clinical predictors of SROT at W24. Single-cell RNA sequencing revealed enrichment of a tumor necrosis factor α signaling via NF-κB signature in CD8+ T cells of patients with no sustained response after TPO-RA discontinuation, which was further confirmed by a significant overexpression of CD69 on CD8+ T cells at baseline in these patients as compared with those achieving SCROT/SROT. Our results strongly support a strategy based on progressive tapering and discontinuation of TPO-RAs for patients with chronic ITP who achieved a stable CR on treatment. This trial was registered at www.clinicaltrials.gov as #NCT03119974.
Introduction
Thrombopoietin receptor agonists (TPO-RAs) represent a paradigm shift in the treatment of adult immune thrombocytopenia (ITP).1 Romiplostim and eltrombopag, the only 2 TPO-RAs available in France, have demonstrated their clinical efficacy with good safety and tolerability in randomized controlled trials and are now widely used as second-line therapy in many countries.2-4 Both bind to the TPO receptor, thus activating the JAK2/STAT5 pathway resulting in increased megakaryocyte proliferation and platelet production.5 ITP results from both an increased destruction of platelets that takes place in the peripheral blood, spleen, or liver, mainly because of a B- and T-cell–mediated autoimmune response to various platelet glycoproteins, notably glycoprotein IIb (GPIIb)/IIIa and GPIb/IX6,7 and a decreased platelet production in the marrow.
Because of their mechanism of action (ie, as growth factors) and the fact that most patients rapidly experience relapse after TPO-RA discontinuation in pivotal trials,2,3 TPO-RAs have long been considered a supportive therapy with no impact on the immune system. However, mainly based on retrospective analysis, we, among other groups, have previously reported durable remissions after TPO-RA discontinuation in ∼20% of patients.8-14 This observation raised the possibility of unexpected immunomodulatory properties of TPO-RAs. Preclinical and in vivo results suggest that 1 mechanism could be the ability of TPO-RAs to restore immune tolerance toward platelet autoantigens, thereby suppressing the autoimmune pathogenesis of ITP.6,15 The mechanism could partly rely on restoring regulatory T-cell (Treg) functions, but the exact mechanism is not fully deciphered. Recently, a prospective study of the progressive tapering of eltrombopag suggested that prolonged remission, >24 weeks after discontinuation, may be achieved in ∼30% of patients with ITP.10 However, the study population included patients with newly diagnosed ITP (ie, <3 months of ITP duration), in whom spontaneous remission may occur regardless of the initial treatment. Therefore, the exact proportion of patients with persistent or chronic ITP at TPO-RA initiation achieving sustained response off treatment (SROT; platelet count >30 × 109/L) or sustained complete response (CR) off treatment (SCROT; platelet count >100 × 109/L) remains to be determined. Moreover, identifying reliable clinical and/or biological markers predicting such remissions remains an unmet need.
The main purpose of this prospective multicenter interventional study is to determine the proportion of SROT at week 24 (W24) and W52 after stopping TPO-RAs in adults with persistent or chronic ITP who previously achieved an initial stable CR to TPO-RAs without receiving any interfering and potentially curative therapies (splenectomy and anti-CD20 treatment). Secondary objectives are to determine the safety of such a strategy, particularly the occurrence of bleeding events, during the study. Finally, we combine single-cell RNA sequencing (scRNA-seq) and flow cytometry before TPO-RA discontinuation to identify some immunological markers that could be associated with SROT.
Patients and methods
Study design
In total, 20 centers from the French ITP reference center network participated in this French nationwide, open prospective, multicenter, interventional study (#NCT03119974).
Patients
Over a 2-year period from September 2017 to February 2020, we enrolled adults (age >18 years) with persistent (3-12 months of ITP duration) or chronic (>12 months of ITP duration) primary ITP, per the international consensus criteria, who experienced stable CR defined by platelet count >100 × 109/L for >2 months on TPO-RAs started from at least 3 months. The main exclusion criteria were concomitant anticoagulation or antiplatelet therapy, previous history of relapse after TPO-RA discontinuation, concomitant treatment with corticosteroids ± IV immunoglobulins, pregnancy or breastfeeding, and rituximab treatment or splenectomy within 2 months before or after TPO-RA initiation.
Ethics
This study was approved by the institutional review board and ethics committee of Ile de France VI (2016-001786-93) and performed in accordance with the Declaration of Helsinki. Oral information was delivered by the investigators, and written consent was obtained from the patient in accordance with the French law.
Data collection
An electronic standardized case report form (Cleanweb, Telemedicine, France) was used to collect data on age, ITP diagnosis, ITP history, platelet count, new bleeding manifestations, ITP status, and treatment initiation/modification at each visit. Each patient had a clinical examination, a bleeding score, and platelet count assessments at W4, W8, W12, W24, W36, and W52. Platelet count was performed weekly until W12, then monthly up to the end of the study (W52). To uniformly assess the severity of bleeding events, each center used a modified bleeding score widely used for adult ITP in France and previously described by our group.16 As compared with the previously described score, age was not considered for this study. Bleeding severity was graded from 1 to 19 based on clinical evaluation, decreased hemoglobin level, and requirement for blood or platelet transfusion. Severe bleeding was arbitrarily defined by a score >8.
TPO-RA tapering and discontinuation
After inclusion, the decrease and weaning of eltrombopag or romiplostim were initiated in accordance with a standardized procedure and timing for each TPO-RA. Eltrombopag was tapered by 25 mg every 2 weeks (for those already at 25 mg per day, eltrombopag had to be stopped at W0) and romiplostim by 1 μg/kg every week until discontinuation. In every case, tapering had to be continued as long as the platelet count remained >30 × 109/L, and TPO-RAs had to be stopped by W10. In case of relapse (bleeding event and/or platelet count decrease <30 × 109/L) during the tapering period or after TPO-RA discontinuation, the decision to increase the dosage of TPO-RA, rechallenge the patient with a TPO-RA, or start a new treatment line was left to every investigator’s discretion.
Study end points
The primary end point was the proportion of patients achieving SROT, defined as platelet count ≥30 × 109/L and no bleeding at W24 after inclusion with no ITP-specific medications. SCROT was defined as a platelet count ≥100 × 109/L and no bleeding with no ITP-specific medications. Patients with a platelet count <30 × 109/L or bleeding before W24 were classified as having a nonsustained response (NSR), as were patients requiring any ITP-specific medication after inclusion. Secondary end points were the SROT and SCROT rates over the study period (W52). Other end points were bleeding events based on the modified French bleeding score and response to a new course of TPO-RAs, in case of relapse.
Ancillary biological studies
At inclusion, whole blood samples were collected from each patient enrolled. Samples were frozen for later analysis. For all patients enrolled, venous blood was collected in heparin or serum tubes (BD Vacutainer) and handled within 24 hours. Serum was obtained after clot removal via centrifugation at 1600 revolutions per minute for 10 minutes and stored at −80°C until use. Peripheral blood mononuclear cells (PBMCs) were isolated via density-gradient sedimentation using Ficoll-Paque (Lymphoprep, Eurobio Scientific). Isolated PBMCs were cryopreserved in 90% heat-inactivated fetal bovine serum supplemented with 10% dimethyl sulfoxide (Gibco). For flow cytometry, cells were thawed using RPMI 1640 (Gibco) and 10% fetal bovine serum (Dutscher), washed twice, and incubated at 1 × 106 PBMCs in 100 μL phosphate-buffered saline with 2% fetal bovine serum for 20 minutes at 4°C, using the LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Thermo Fisher Scientific) or Zombie Violet Fixable Viability Kit (BioLegend) and conjugated antibodies listed in supplemental Method Table 1, which is available on the Blood website. Cells were acquired on an LSR Fortessa Analyzer (BD Bioscience), and data analysis was performed using Kaluza version 2.1 (Beckman Coulter). Circulating T- and B-cell subpopulations were analyzed using a flow cytometry panel. Anti-platelet antibodies were detected by using the indirect specific immobilization of platelet antigenassay (apDia, Turnhout, Belgium).
Antigen-reactive T-cell assay
We adapted the assay from the originally described antigen-reactive T-cell enrichment procedure17 and activation-induced marker assay.18,19 In brief, ∼0.5 × 107 to 4 × 107 cryopreserved PBMCs were plated in RPMI 1640 medium (Gibco), supplemented with 5% human AB serum (Sigma Aldrich, Schnelldorf, Germany), and stimulated with GPIIb/IIIa peptides or dimethyl sulfoxide alone for 24 hours in the presence of 1 mg/mL CD40 and 1 mg/mL CD28 pure antibody (both from Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were labeled with CD154-biotin and CD137-PE, and anti-biotin (CD154 MicroBead Kit, Miltenyi Biotec) and anti-PE (CD137 MicroBead Kit, Miltenyi Biotec) antibodies were added, then were magnetically enriched by using MS Columns (Miltenyi Biotec). Surface staining was performed on flushed-out fractions with the magnetically labeled cells. The frequency of antigen-specific T cells was determined based on the count of CD154+OX40+ or CD137 OX40+ T cells after enrichment, normalized to the total number of CD4+ T cells applied on the column. For each stimulation, background cells enriched from the nonstimulated control were subtracted.
scRNA-seq library preparation, sequencing, and gene expression analysis
scRNA-seq analyses were performed on frozen PBMCs isolated from heparin blood samples from 8 patients at baseline (4 with SCROT and 4 with NSR; supplemental Method Table 2). PBMCs were thawed per the 10X Genomics protocol. The RNA-seq libraries were generated by using the Chromium Next GEM Single Cell V(D)J Reagent Kit version 1.1 (10X Genomics), per the manufacturer’s protocol at Institut Imagine (INSERM UMR1163; supplemental Methods).
Cytokines study
Serum concentrations of tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), IL-8, and IL-6 were assessed on an Ella Platform (protein simple, Bio-Techne), which is a rapid cytokine detection system based on 4 parallel single-plex microfluidics enzyme-linked immunosorbent assays run in triplicate within cartridges with the SPCKA-PS-003229 Kit, following the manufacturer’s instructions. Serum concentrations of perforin and granzyme A were measured using enzyme-linked immunosorbent assay (Invitrogen), following the manufacturer’s recommendations.
Sample size calculation
From previous findings obtained in retrospective series reported by our group and others, we postulated a 25% response at W24 (minimal response, 15% and maximal response, 30%).10 With a 1-sided 5% α risk and power of 80%, 43 patients were needed. At least 11 successes observed at W24 fulfilled the prespecified hypothesis. Considering that 10% of patients were lost to follow-up or were nonevaluable, we aimed to include 48 patients.20
Statistical analysis
Qualitative data are expressed as number (percentages), and quantitative data as the median (interquartile range [IQR]). The main and secondary end point analyses were based on the intention-to-treat principle. The SROT and SCROT at W24 after inclusion were assessed based on number (percentage) and 95% confidence intervals (CIs) with a binomial distribution. Secondary end points were assessed using the same methodology for categorical end points and by median (IQR) for quantitative end points. The incidence of SROT and SCROT over 52 weeks after TPO-RA discontinuation was expressed as survival rates and 95% CIs, with survival curves based on the Kaplan-Meier method. The association of baseline characteristics and relapse after TPO-RA discontinuation at W24 and W52 was assessed using a χ2 or Fisher exact test for categorical variables and the Wilcoxon-Mann-Whitney test for quantitative variables. Unadjusted odds ratios (ORs) and their 95% CIs were estimated using a univariate logistic regression model. Pairwise analyses were used to assess confounding factors and interactions were sought. Missing data were replaced with multiple imputation. We used the multiple-multivariate-imputation-by-chained-equations procedure with the missing-at-random assumption. Baseline characteristics were used to impute the missing data values, and we independently analyzed 20 copies of the data. All tests were 2-tailed and P < .05 was considered statistically significant. Analyses involved using STATA version 17.0 (StataCorp, College Station, TX).
Results
Patient characteristics
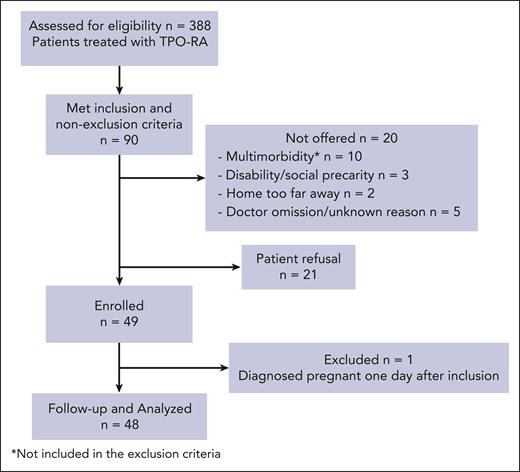
Between September 2017 and February 2020, 388 patients treated with TPO-RAs were assessed for eligibility, and 93 met the inclusion criteria. Among them, 21 declined to participate, and 20 were not offered to participate (10 because of various underlying comorbidities, 3 because of disability/social precarity, 2 because they lived too far away from the participating centers, and 5 because of doctor omission or an unknown reason; Figure 1). Eventually, 49 patients were enrolled. One patient was excluded because she was found to be pregnant 1 day after inclusion. Overall, 48 patients, 29 (60.5%) females, with a median age of 58.5 years (IQR, 41-73.5 years) were included (Table 1). At ITP diagnosis, 27 of 48 patients (56.3%) had a reported bleeding manifestation, with a median modified French bleeding score of 3 (IQR, 2-7). At TPO-RA initiation, 22 of 48 patients (47%) had previously received >2 treatment lines for ITP, 22 of 48 (46%) had received rituximab (median, 0.69 years; IQR, 0.51-3.30) >6 months before TPO-RA initiation for 16 of 22 (73%), and 6 of 48 (13%) had a history of splenectomy (median, 2.36 years; IQR, 0.70-4.33). ITP was persistent (3-12 months) for 18 of 48 patients (37%) (including 8 [44%] with ITP duration >6 months) and chronic (>12 months) for 30 of 48 (63%). The median maximal platelet count achieved on TPO-RA was 291 × 109/L (range, 215 × 109/L to 408 × 109/L). At inclusion, 40 patients (83%) were on eltrombopag, and 8 (17%) on romiplostim, with a median duration of TPO-RA exposure of 1.6 years (IQR, 1.0-3.8 years).
Flowchart of patient selection. ∗Not included in the exclusion criteria.
Demographics and ITP characteristics at inclusion
| . | Total (n = 48)∗ . |
|---|---|
| Female, n (%) | 29 (60.5) |
| Age, median (IQR), y | 58.5 (41-73.5) |
| ITP previous treatment, n (%) | |
| Corticosteroids and/or IV immunoglobulin | 48 (100) |
| Splenectomy | 6 (13) |
| Rituximab | 22 (46) |
| Dapsone/danazol | 23 (48) |
| >2 therapeutic lines | 22 (47) |
| Previous treatment with another TPO-RA† | 14 (29) |
| ITP duration at TPO-RA initiation, n (%) | |
| Persistent | 18 (37) |
| Chronic | 30 (63) |
| ITP duration at inclusion (starting TPO-RA discontinuation) | |
| Persistent, n (%) | 2 (4) |
| Chronic, n (%) | 46 (96) |
| Median (IQR), y | 6 (2.8-10) |
| TPO-RA drug class at inclusion | |
| Romiplostim, n (%) | 8 (17) |
| Weekly dose, μg/kg × body weight, median (IQR), (n = 7) | 4 (3-6) |
| Eltrombopag, n (%) | 40 (83) |
| Daily dose, median (IQR), mg (n = 40) | 50 (25-50) |
| TPO-RA duration, median (IQR), y (n = 46) | 1.6 (1.0-3.8) |
| Maximal platelet count under TPO-RAs treatment, × 109/L (IQR) | 291 (215-408) |
| . | Total (n = 48)∗ . |
|---|---|
| Female, n (%) | 29 (60.5) |
| Age, median (IQR), y | 58.5 (41-73.5) |
| ITP previous treatment, n (%) | |
| Corticosteroids and/or IV immunoglobulin | 48 (100) |
| Splenectomy | 6 (13) |
| Rituximab | 22 (46) |
| Dapsone/danazol | 23 (48) |
| >2 therapeutic lines | 22 (47) |
| Previous treatment with another TPO-RA† | 14 (29) |
| ITP duration at TPO-RA initiation, n (%) | |
| Persistent | 18 (37) |
| Chronic | 30 (63) |
| ITP duration at inclusion (starting TPO-RA discontinuation) | |
| Persistent, n (%) | 2 (4) |
| Chronic, n (%) | 46 (96) |
| Median (IQR), y | 6 (2.8-10) |
| TPO-RA drug class at inclusion | |
| Romiplostim, n (%) | 8 (17) |
| Weekly dose, μg/kg × body weight, median (IQR), (n = 7) | 4 (3-6) |
| Eltrombopag, n (%) | 40 (83) |
| Daily dose, median (IQR), mg (n = 40) | 50 (25-50) |
| TPO-RA duration, median (IQR), y (n = 46) | 1.6 (1.0-3.8) |
| Maximal platelet count under TPO-RAs treatment, × 109/L (IQR) | 291 (215-408) |
n = 48, unless otherwise specified.
Switch to another TPO-RA agent without discontinuation.
Overall response after TPO-RA discontinuation
As per the protocol, eltrombopag was immediately discontinued for 10 of 15 patients who were receiving 25 mg per day but was slightly delayed for 5 patients essentially because of the patient’s decision (W1, n = 2; W2, n = 2; W4, n = 2). For those at 50 mg (n = 17), eltrombopag was decreased for 11 patients at W2, as planned, and slightly delayed for 6 (W4, n = 3; W6, n = 1; W8, n = 1). There was no protocol deviation for patients on 75 mg eltrombopag (n = 7) or romiplostim (n = 8).
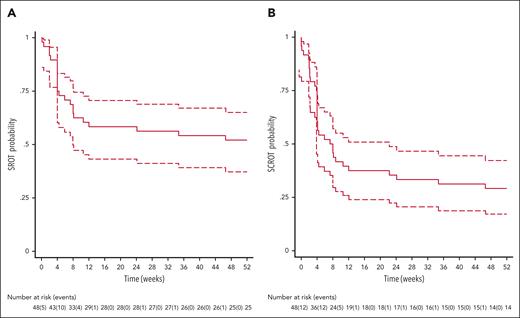
In the intention-to-treat analysis, at W24, 27 of 48 patients (56.2%; 95% CI, 41.2-70.5) achieved SROT (primary end point), and 15 of 48 (31.3%; 95% CI, 18.9-44.5) achieved SCROT. At W52, 25 of 48 patients (52.1%; 95% CI, 37.2-66.7) achieved SROT, and 14 of 48 (29.2%; 95% CI, 17.2-42.3) achieved SCROT. For patients who relapsed during follow-up, the median time to relapse after TPO-RA’s discontinuation was 4 weeks (IQR, 4-8.1 weeks; Figure 2).
Relapse-free probability of SROT and SCROT after TPO-RA discontinuation. Kaplan-Meier curves for relapse-free probability of SROT (A) and SCROT (B) after TPO-RA discontinuation (W0).
Relapse-free probability of SROT and SCROT after TPO-RA discontinuation. Kaplan-Meier curves for relapse-free probability of SROT (A) and SCROT (B) after TPO-RA discontinuation (W0).
Bleeding and response to a new course of TPO-RA
Among patients who relapsed by W24 (n = 21) or W52 (n = 23), bleeding events occurred in 12 of 21 (57.1%) and 14 of 23 patients (60.9%), respectively, with a median platelet count of 31 × 109/L (IQR, from 26 × 109/L to 39 × 109/L) and 31 × 109/L (IQR, from 23 × 109/L to 39 × 109/L). No severe bleeding episodes, defined by a modified French bleeding score >8 occurred at time of relapse (supplemental Table 1). In total, 12 of 21 patients with a bleeding event at relapse before W24 were rechallenged with the same TPO-RA, and 11 of 12 (91.7%) showed CR after a median time of 2 weeks (IQR, 2-4 weeks). Among the 2 patients who relapsed after W24, 1 received corticosteroids and rituximab, and 1 received a new course of TPO-RA at W52.
Risk factors of relapse after TPO-RA discontinuation
Upon univariable analysis, at W24, the probability of NSR (bleeding event and/or platelet count <30 × 109/L) tended to be associated with a platelet count <150 × 109/L at inclusion (P = .07; Table 2). There was no association with previous treatment lines. At W52, the probability of NSR tended to be associated with chronic ITP at TPO-RA initiation (P = .082; supplemental Table 2). When considering SCROT, the probability of not achieving a CR at W24 and W52 was associated with chronic ITP at TPO-RA initiation (OR, 5.01; 95% CI; 1.30-19.35; P = .019; and OR, 6.44; 95% CI, 1.56-26.67; P = .01, respectively; supplemental Tables 3 and 4). Similar results were obtained with nonimputed data (not shown).
Risk factors of NSR (W24)
| . | Total (n = 48)∗ . | SROT (n = 27) . | NSR (n = 21) . | Univariable analysis . | |
|---|---|---|---|---|---|
| Unadjusted—OR [95% CI] . | P value† . | ||||
| Age, median (IQR), y | 58.5 (41-73.5) | 58 (34-74) | 60 (51-71) | 1.01 [0.98-1.04] | .69 |
| No. of therapeutic lines before TPO-RAs, n = 48 | .98 | ||||
| 1 | 11 (23.0) | 6 (22.2) | 5 (23.8) | 1 (reference) | |
| ≥2 | 37 (77.0) | 21 (77.8) | 16 (76.2) | 0.98 [0.25-3.81] | |
| Splenectomy history | 6 (12.5) | 2 (7.4) | 4 (19.0) | 2.88 [0.47-17.54] | .25 |
| Previous treatment with rituximab | 22 (45.8) | 12 (44.4) | 10 (47.6) | 1.12 [0.36-3.53] | .85 |
| Previous treatment with another TPO-RA | 14 (29.2) | 6 (22.2) | 8 (38.1) | 2.44 [0.66-9.07] | .18 |
| ITP duration at TPO-RA initiation | .19 | ||||
| Persistent | 18 (37.5) | 12 (44.4) | 6 (28.6) | 1 (reference) | |
| Chronic | 30 (62.5) | 15 (55.6) | 15 (71.4) | 2.30 [0.66-8.01] | |
| ITP duration at inclusion | .87 | ||||
| Persistent | 2 (4.2) | 1 (3.7) | 1 (4.8) | 1 (reference) | |
| Chronic | 46 (95.8) | 26 (96.3) | 20 (95.2) | 0.77 [0.05-13.07] | |
| Platelet count >150 × 109/L at inclusion | 34 (70.8) | 22 (81.5) | 12 (57.1) | 0.30 [0.08-1.11] | .07 |
| TPO-RA duration, median [IQR], y (n = 46) | 1.6 [1.0-3.8] | 2.0 [1.1-3.8] | 1.2 [0.9-3.2] | 0.95 [0.74-1.23] | .33 |
| TPO-RA drug class | .115 | ||||
| Eltrombopag | 40 (83.3) | 25 (92.6) | 15 (71.4) | 1 (reference) | |
| Romiplostim | 8 (16.7) | 2 (7.4) | 6 (28.6) | 5.00 [0.89-28.02] | |
| . | Total (n = 48)∗ . | SROT (n = 27) . | NSR (n = 21) . | Univariable analysis . | |
|---|---|---|---|---|---|
| Unadjusted—OR [95% CI] . | P value† . | ||||
| Age, median (IQR), y | 58.5 (41-73.5) | 58 (34-74) | 60 (51-71) | 1.01 [0.98-1.04] | .69 |
| No. of therapeutic lines before TPO-RAs, n = 48 | .98 | ||||
| 1 | 11 (23.0) | 6 (22.2) | 5 (23.8) | 1 (reference) | |
| ≥2 | 37 (77.0) | 21 (77.8) | 16 (76.2) | 0.98 [0.25-3.81] | |
| Splenectomy history | 6 (12.5) | 2 (7.4) | 4 (19.0) | 2.88 [0.47-17.54] | .25 |
| Previous treatment with rituximab | 22 (45.8) | 12 (44.4) | 10 (47.6) | 1.12 [0.36-3.53] | .85 |
| Previous treatment with another TPO-RA | 14 (29.2) | 6 (22.2) | 8 (38.1) | 2.44 [0.66-9.07] | .18 |
| ITP duration at TPO-RA initiation | .19 | ||||
| Persistent | 18 (37.5) | 12 (44.4) | 6 (28.6) | 1 (reference) | |
| Chronic | 30 (62.5) | 15 (55.6) | 15 (71.4) | 2.30 [0.66-8.01] | |
| ITP duration at inclusion | .87 | ||||
| Persistent | 2 (4.2) | 1 (3.7) | 1 (4.8) | 1 (reference) | |
| Chronic | 46 (95.8) | 26 (96.3) | 20 (95.2) | 0.77 [0.05-13.07] | |
| Platelet count >150 × 109/L at inclusion | 34 (70.8) | 22 (81.5) | 12 (57.1) | 0.30 [0.08-1.11] | .07 |
| TPO-RA duration, median [IQR], y (n = 46) | 1.6 [1.0-3.8] | 2.0 [1.1-3.8] | 1.2 [0.9-3.2] | 0.95 [0.74-1.23] | .33 |
| TPO-RA drug class | .115 | ||||
| Eltrombopag | 40 (83.3) | 25 (92.6) | 15 (71.4) | 1 (reference) | |
| Romiplostim | 8 (16.7) | 2 (7.4) | 6 (28.6) | 5.00 [0.89-28.02] | |
All results are given as a number of events (in percentage), unless otherwise specified.
n = 48, unless otherwise specified.
χ2 test or Fisher exact test for categorical data and Wilcoxon-Mann-Whitney test for quantitative data.
Immunological biomarkers associated with relapse after TPO-RA discontinuation
We hypothesized that a signature of the autoimmune process may be detectable before TPO-RA discontinuation among patients with an eventual relapse. We first performed scRNA-seq with 10X Genomics technology of PBMCs from 8 patients at inclusion (W0): 4 with maintained SCROT (>100 × 109/L) at W24 and 4 with NSR at W24. In parallel, we used a flow cytometry panel to analyze the distribution and phenotype of T and B cells, previously reported to be dysregulated in ITP in 36 patients (supplemental Figure 1). Unsupervised clustering analysis of scRNA-seq showed 15 cell clusters divided based on their gene expression profiles, representing the major T-, B-, and myeloid cell subpopulations (Figure 3A). All clusters were similarly represented in each patient group (Figure 3B). Similar results were obtained by using multiparametric fluorescence-activated cell sorting analysis at baseline before TPO-RA discontinuation in patients classified based their response status at W24 (supplemental Figure 1).
Multiparameter profiling of the peripheral blood for patients with ITP before TPO-RA discontinuation. (A) Uniform manifold approximation and projection (UMAP) and clustering of 45 548 single cells from 8 patients at baseline (n = 4 with SCROT and n = 4 with NSR) based on scRNA-seq results. Cell type labels were assigned to clusters based on a manually curated list of marker genes. (B) Relative cluster distribution of all cells from SCROT and NSR donor groups. Bar indicates median. (C-D) Number of CD19+ B cells and CD19+CD27+IgD−CD38− memory B cells (MBCs) assessed via flow cytometry at baseline for patients with SCROT, SROT, and NSR. (E-F) Percentages of CD27+CD38int/+CD71+ activated B cells (ABCs) and CD27highCD38high antibody–secreting cells (ASCs) assessed via flow cytometry at baseline for patients with SCROT, SROT, and NSR. (G) Optical densities (ODs) of anti-GPIIbIIIa antibodies (Abs) measured using specific immobilization of platelet antigen assay at baseline for patients with SCROT, SROT, and NSR. (H-K) Percentages of CD3+CD4+CCR7−CD45RA− memory (TEM), CD3+CD4+CCR7+CD45RA− central memory (TCM), CD3+CD4+CXCR5+ICOS+PD-1+ circulating T-follicular helper (cTFH), and CD3+CD4+CD25highCD127low Treg CD4+ at baseline for patients with SCROT, SROT, and NSR. (L) Frequency of GPIIbIIIa-specific CD4+ T cells assessed via flow cytometry, with an adapted antigen-reactive T-cell enrichment technology at baseline for patients with SCROT (platelet count >100 × 109/L, SROT [platelet count 30 × 109/L to 100 × 109/L], and NSR [platelet count <30 × 109/L and or bleeding]). Bars indicate median. Kruskal-Wallis and Wilcoxon-Mann-Whitney tests were used as appropriate. Mono, monocyte; NK, natural killer; pDCs, plasmacytoid dendritic cells; RBCs, red blood cells.
Multiparameter profiling of the peripheral blood for patients with ITP before TPO-RA discontinuation. (A) Uniform manifold approximation and projection (UMAP) and clustering of 45 548 single cells from 8 patients at baseline (n = 4 with SCROT and n = 4 with NSR) based on scRNA-seq results. Cell type labels were assigned to clusters based on a manually curated list of marker genes. (B) Relative cluster distribution of all cells from SCROT and NSR donor groups. Bar indicates median. (C-D) Number of CD19+ B cells and CD19+CD27+IgD−CD38− memory B cells (MBCs) assessed via flow cytometry at baseline for patients with SCROT, SROT, and NSR. (E-F) Percentages of CD27+CD38int/+CD71+ activated B cells (ABCs) and CD27highCD38high antibody–secreting cells (ASCs) assessed via flow cytometry at baseline for patients with SCROT, SROT, and NSR. (G) Optical densities (ODs) of anti-GPIIbIIIa antibodies (Abs) measured using specific immobilization of platelet antigen assay at baseline for patients with SCROT, SROT, and NSR. (H-K) Percentages of CD3+CD4+CCR7−CD45RA− memory (TEM), CD3+CD4+CCR7+CD45RA− central memory (TCM), CD3+CD4+CXCR5+ICOS+PD-1+ circulating T-follicular helper (cTFH), and CD3+CD4+CD25highCD127low Treg CD4+ at baseline for patients with SCROT, SROT, and NSR. (L) Frequency of GPIIbIIIa-specific CD4+ T cells assessed via flow cytometry, with an adapted antigen-reactive T-cell enrichment technology at baseline for patients with SCROT (platelet count >100 × 109/L, SROT [platelet count 30 × 109/L to 100 × 109/L], and NSR [platelet count <30 × 109/L and or bleeding]). Bars indicate median. Kruskal-Wallis and Wilcoxon-Mann-Whitney tests were used as appropriate. Mono, monocyte; NK, natural killer; pDCs, plasmacytoid dendritic cells; RBCs, red blood cells.
We observed no significant homeostatic changes in the distribution of B-cell subsets, including CD27+CD38int/+CD71+–activated B cells and CD27highCD38high antibody–secreting cells (Figure 3C-F; supplemental Figure 2A-B). Anti-GpIIb/IIIa/IbIX antibodies measured using an indirect specific immobilization of platelet antigen assay did not differ between the groups (Figure 3G). The distributions of T-cell subsets (supplemental Figure 2C-E), including CCR7+CD45RA+-naive, CCR7−CD45RA−-memory, CCR7+CD45RA−–central memory CD4+ and CD8+ T cells (Figure 3H-I) as well as CD4+CXCR5+ICOS+PD-1+ circulating T-follicular helper cells (Figure 3J) and CD4+CD25highCD127low Tregs (Figure 3K) were also comparable between the groups.
We also investigated the proportion of antiplatelet reactive CD4+ T cells via flow cytometry with the antigen-reactive T-cell enrichment technology for patients at baseline. We generated a pool of 32 peptides for the GPIIbIIIa antigen by using the major histocompatibility II peptide–binding prediction tool TepiTool (IEDB Analysis Resource) and known peptides.21,22 GPIIbIIIa-specific CD4+ T cells were identified in 5 of 8 patients with NSR, but we also observed GPIIbIIIa-specific CD4+ T cells in 2 of 7 with SCROT at W24, which suggests an immunological heterogeneity in these patients (supplemental Figure 2F; Figure 3L).
Next, we compared the transcriptional signature of patients with SCROT and NSR at W24 for each scRNA-seq cell cluster, focusing on the most-represented clusters. Gene set enrichment analysis23 revealed enrichment of several immune pathways (Figure 4A). We observed clear enrichment of a TNFα signaling via NF-κB pathway in CD8+ memory T-cell clusters (Figure 4A-B) and, to a lesser extent, in innate-like T cells, natural killer cells, and CD4+ memory T-cell clusters. However, this gene signature was heterogenous among patient groups (supplemental Figure 3A). Among the differentially expressed genes in CD8+ memory T-cell clusters between patients with NSR and SCROT belonging to the TNF-α signaling via NF-κB pathway (Figure 4C), CD69, an early T-cell activation marker,24 was found to be significantly upregulated among patients with NSR. Indeed, although the number of CD8+ T cells expressing CD69 transcripts did not differ between patients with SCROT (57%) and NSR (67%), the differential gene expression was clearly higher in patients with NSR (adjusted P = 8,63.10−22). Because CD69 was the only surface protein marker overexpressed in the TNFα signaling via NF-κB signature, we assessed its expression in 22 patients with available PBMCs. CD69 expression on CD8+ T cells after overnight resting of PBMCs was significantly higher at baseline in patients with NSR than those with SCROT, which supports the relevance of this signature (Figure 4D). Intragroup heterogeneity was high, as previously observed in our smaller scRNA-seq data set. CD69 showed greater expression, although not significantly, on CD4+ cells from patients with NSR (Figure 4E). Similar results were obtained after immediate staining of PBMCs (supplemental Figure 3B-C). We found neither a correlation between CD8+CD69+ T-cell number and ITP duration (supplemental Figure 3D) nor with previous response to corticosteroids (not shown). Finally, CD69 was overexpressed on CD8+ T cells of patients sampled at relapse (n = 8; median, 12 weeks; range, 8-23 weeks after starting discontinuation) but not those with SCROT sampled at W24 (n = 8; supplemental Figure 3E). Serum levels of TNFα and other inflammatory cytokines such as IL-1β, IL-6, and IL-8 did not strongly differ among patients with SCROT, SROT, and NSR (supplemental Figure 3F-I) nor did levels of perforin and granzyme B, indirect soluble markers of cytotoxic lymphocytes (supplemental Figure 3H-I). Altogether, these findings strongly suggest a global level of immune activation in patients showing relapse after TPO-RA discontinuation, which can be detected by the CD69 expression on CD8+ T cells.
Differential gene expression analysis identifies CD69 expression in CD8 T cells as a predictor of NSR. (A) Plot showing the preranked gene set enrichment analysis results for comparison of patients with SCROT vs those with NSR in lymphoid cells and CD14+ monocytes clusters. Dot size represents the normalized enrichment score (NES). Dot color represents the sign of the log fold change ∗ log10(false discovery rate [FDR] value) (ie, blue indicates enrichment in patients with SCROT and red indicates enrichment in patients with NSR). Only gene sets with NES >1.5 and FDR <0.05 in at least 1 of the comparisons are shown. (B) Violin plot of TNF-α signaling via NF-κB pathway signature in CD8+ memory T cells from patients with SCROT and NSR at baseline (left). Enrichment plots for TNF-α signaling via NF-κB gene set in the comparison of patients with SCROT vs those with NSR (right). (C) Dot plots showing expression of expressed genes from TNF-α signaling via NF-κB gene set in CD8+ memory T cells from patients with SCROT and NSR at baseline. Dot sizes represent the percentage of cluster cells in which transcripts for that gene are detected. Dot color represents the average expression level (scaled normalized counts) of that gene in the population. Percentages of CD3+CD8+CD69+ (D) or CD3+CD4+CD69+ (E) T cells assessed via flow cytometry after overnight resting at baseline for patients with SCROT, SROT, and NSR. Bars indicate median. The Kruskal-Wallis and Wilcoxon-Mann-Whitney tests were used as appropriate. Only significant P values are reported; ∗∗P < .01.
Differential gene expression analysis identifies CD69 expression in CD8 T cells as a predictor of NSR. (A) Plot showing the preranked gene set enrichment analysis results for comparison of patients with SCROT vs those with NSR in lymphoid cells and CD14+ monocytes clusters. Dot size represents the normalized enrichment score (NES). Dot color represents the sign of the log fold change ∗ log10(false discovery rate [FDR] value) (ie, blue indicates enrichment in patients with SCROT and red indicates enrichment in patients with NSR). Only gene sets with NES >1.5 and FDR <0.05 in at least 1 of the comparisons are shown. (B) Violin plot of TNF-α signaling via NF-κB pathway signature in CD8+ memory T cells from patients with SCROT and NSR at baseline (left). Enrichment plots for TNF-α signaling via NF-κB gene set in the comparison of patients with SCROT vs those with NSR (right). (C) Dot plots showing expression of expressed genes from TNF-α signaling via NF-κB gene set in CD8+ memory T cells from patients with SCROT and NSR at baseline. Dot sizes represent the percentage of cluster cells in which transcripts for that gene are detected. Dot color represents the average expression level (scaled normalized counts) of that gene in the population. Percentages of CD3+CD8+CD69+ (D) or CD3+CD4+CD69+ (E) T cells assessed via flow cytometry after overnight resting at baseline for patients with SCROT, SROT, and NSR. Bars indicate median. The Kruskal-Wallis and Wilcoxon-Mann-Whitney tests were used as appropriate. Only significant P values are reported; ∗∗P < .01.
Discussion
This prospective study shows an unexpectedly high rate of SROT after TPO-RA discontinuation among patients with persistent or chronic ITP who achieved a stable CR on TPO-RAs. Moreover, we did not observe any severe bleeding manifestation among patients who relapsed after TPO-RA discontinuation, and all but 1 patient achieved CR after TPO-RA rechallenge. This reassuring finding is consistent with that from a previous study showing that repeated short courses of eltrombopag do not increase the risk of bleeding due to rebound thrombocytopenia when patients are carefully monitored by the physician.25 Of note, none of the patients in our study had a history of severe bleeding. When relapse occurred, it was mainly observed within the first few weeks after discontinuation.
Sustained remission after romiplostim tapering has been reported in approximately one-third of patients with newly diagnosed ITP.13 The recent phase 2 multicenter, uncontrolled prospective Italian study showed that 25% of adults with newly or persistent ITP achieved SROT for at least 6 months after TPO-RA discontinuation10 However, in the absence of a control group, it is difficult to affirm that these SROTs could not be because of spontaneous remission, which may sometimes occur during the initial or early persistent phase of the disease.26 Our study was also uncontrolled, but its strength is that only patients with persistent or mostly chronic ITP at the time of TPO-RA initiation were included.
The SROT rate of this study is higher than that previously reported, with slightly >50% of patients requiring no further treatment 1 year after TPO-RA discontinuation. Several parameters may explain these differences. Firstly, we selected patients with ITP achieving a stable CR on TPO-RAs. Accordingly, in the Italian study, the SROT rate was also ∼50% among patients who started to taper TPO-RAs while being in complete remission. Secondly, the duration of TPO-RA treatment, which was >1 year before discontinuation in many of our patients, might have played an important role in restoring immune tolerance toward autologous platelets. Prolonged treatment may be required to induce the immunomodulatory effects of TPO-RAs in chronic ITP. Determining the minimal duration of treatment required to achieve such immunomodulation is difficult to address in part because the mechanisms by which TPO-RAs can induce durable remission are far from being elucidated. TPO-RAs have been suggested to restore Treg levels and function in the peripheral blood or spleen.15 The main hypothesis is that restoring a healthy platelet mass with TPO-RAs may promote the expansion of Treg cells through the increased expression of transforming growth factor β,15 but platelets may also interact with many other components of the immune system.27 Thus, improving our understanding of the immunoregulatory properties of platelets and the impact of prolonged exposure to platelet antigens on the immune system in patients receiving TPO-RAs remains a future challenge.
One potential limitation of this study could be a selection bias because, in most participating centers, some patients did not agree to discontinue TPO-RA (23%), and therefore were not included. Moreover, CR is on average achieved in ∼50% of patients with ITP treated with TPO-RAs,10 so our results cannot be extrapolated to patients achieving a platelet count between 30 × 109/L and 100 × 109/L on TPO-RA. Based on our findings, considering the cost of these drugs, tapering and discontinuing TPO-RA should be considered when platelet counts are >100 × 109/L and have been maintained for at least 6 to 12 months. Other prospective studies assessing this strategy are ongoing (#NCT03524612).
Although this study was specifically designed to address an immunomodulatory effect of TPO-RA by excluding newly diagnosed ITP, in which spontaneous remission may occur, 12% of the patients had ITP for 3 to 6 months at TPO-RA initiation. Thus, we cannot fully exclude that a spontaneous remission could have occurred anyway in a few of them. The inclusion criteria required that TPO-RA be initiated at least 2 months after rituximab because delayed response beyond this period are quite uncommon with rituximab.28,29 Approximately half of the patients had previously received rituximab, but in most of them, this period was >6 months. Therefore, we found no statistical trend to support a potential bias induced by prior rituximab treatment. There was also no significant association with the history of splenectomy, which was performed for most of them at least 1 year before TPO-RA initiation. Finally, most patients were on eltrombopag, so a specific study with romiplostim is probably needed. Whether these results could be extended to other TPO-RA agents such as avatrombopag and lusutrombopag remains to be confirmed, but both are small molecules that bind to the transmembrane region of the TPO receptor (c-Mpl) in a similar manner to eltrombopag, which may result in a similar effect.
The goal of our exploratory ancillary study was not to decipher immunological changes induced by TPO-RAs or help identify molecular mechanisms responsible for long-term remission after TPO-RA discontinuation but rather to assess potential differences in circulating lymphoid cells that would predict relapse. scRNA-seq and flow cytometry revealed no substantial changes in immune subpopulation frequencies. However, transcriptional expression analysis, albeit heterogenous, and performed with a limited number of patients, revealed the strong enrichment of a TNFα signaling via NF-κB signature in CD8+ T cells of patients with NSR after TPO-RA discontinuation. Increased serum/plasma levels of TNF have been reported in patients with ITP30,31 and in CD4+ T cells from patients with chronic ITP.32 Treatment with TNF blockers was also found effective in a small group of patients with ITP.33,34 However, in our study, the level of TNFα did not help predict the clinical outcome. So, this signature, which can be driven by diverse immune ligands or cytokines, may reflect a more global activation of the immune system than systemic chronic inflammation.35
Regardless, this allowed us to identify that the early activation marker CD69 was overexpressed on CD8+ T cells in a significant proportion of patients with NSR but almost no patients with SCROT. The expression of CD69 on CD8+ T cells could result from a platelet-specific CD8+ T-cell response and/or from bystander activation by the microenvironment. Although CD8+ T cells have been implicated as a mechanism of platelet destruction, the current evidence for the existence of platelet-specific CD8+ T cells in ITP remains controversial.36-40 Several arguments support the hypothesis of bystander activation. Firstly, CD69 was also expressed at the surface of CD4+ T cells in our study, which suggests that activation is not restricted to CD8+ cells. Secondly, we observed CD69 overexpression on a large fraction of CD8+ T cells from patients with NSR after several hours of T-cell culture without any antigenic stimulation. Thirdly, memory CD8+ T cells harbored no transcriptional hallmark of proliferation, which would have been expected in response to specific antigenic stimulation. Finally, the levels of perforin and granzyme B, indirect soluble markers of cytotoxic lymphocytes, were not increased in patients with NSR. Of note, the CD8+CD69+ cell percentage was found higher in children with ITP than in healthy donors.41 Overall, the presence of activated CD8+CD69+ T cells likely reflects the underlying active immune response in patients poised for relapse after TPO-RA discontinuation. Although promising, the use of CD8+CD69+ T cells as a predictor of relapse needs to be validated in a larger cohort of patients, and the precise role of these CD8+ T cells needs to be elucidated.
In conclusion, our study strongly supports a strategy of progressive tapering and weaning of TPO-RAs for adults with persistent or chronic ITP who achieve a stable CR on TPO-RAs. Overexpression of CD8+CD69+ T cells at the time of tapering TPO-RAs could be an interesting predictor of relapse.
Acknowledgments
This work was supported by a grant from Programme Hospitalier de Recherche Clinique (PHRC 2016). The sponsor was Assistance Publique–Hôpitaux de Paris (Département de la Recherche Clinique et du Développement). The ancillary study was funded by Filière de Santé Maladie Rare Immuno-Hématologique, Paris (MARIH). E.C. was supported by a Poste d'Accueil APHP.
Authorship
Contribution: M. Mahévas, B.G., and M. Michel conceptualized the study; S.G., E.C., and M. Mahévas curated the data; S.G., E.C., I.A., P.C., M. Menager, E.B., F.C.-P., and B.G. performed formal analysis; M. Mahévas and B.G. acquired funding; J.-F.V., D.G., M. Malphettes, S.C., F.L., S.A., B.B., O.L., N.N., O.F., G.M., M.H., M.G.-V., J.-P.M., L.T., N.M., A.-S.M., A.P., T.L.G., F.R.-P., A.R., N.L., M. Michel, B.G., and M. Mahévas provided patients; S.G., E.C., and M. Mahévas performed methodology; S.G., E.C., M. Mahévas, and B.G. administered the project; M. Mahévas and B.G. provided resources; P.C. performed software; M. Mahévas and B.G. supervised the study; S.G., E.C., and M. Mahévas visualized the study; S.G., E.C., M. Mahévas, and B.G. wrote the first draft of the manuscript; and all authors wrote, reviewed, and edited the manuscript.
Conflict-of-interest disclosure: M. Mahévas received funds for research from GSK and fees from Amgen and Novartis for lectures. B.G. served as an expert for Amgen, Novartis, Grifols, and Sobi. M. Michel received honoraria (advisory boards and speaker fees) from Novartis, Amgen, UCB, Argenx, Alexion, and Sanofi. E.C. received honoraria (advisory boards and speaker fees) from Novartis, UCB, and Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Matthieu Mahévas, Department of Internal Medicine, Henri Mondor University Hospital, Assistance Publique-Hôpitaux de Paris, 51 avenue du Marechal de Lattre de Tassigny, 94000 Créteil, France; e-mail: matthieu.mahevas@aphp.fr.
References
Author notes
∗S.G. and E.C. contributed equally to this study.
†I.A., P.C., and E.B. contributed equally to this study.
RNA sequencing data reported in this article have been deposited in the ArrayExpress database at www.ebi.ac.uk/arrayexpress (accession number E-MTAB-12397).
This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available on request from the corresponding author, Matthieu Mahévas (matthieu.mahevas@aphp.fr).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Multiparameter profiling of the peripheral blood for patients with ITP before TPO-RA discontinuation. (A) Uniform manifold approximation and projection (UMAP) and clustering of 45 548 single cells from 8 patients at baseline (n = 4 with SCROT and n = 4 with NSR) based on scRNA-seq results. Cell type labels were assigned to clusters based on a manually curated list of marker genes. (B) Relative cluster distribution of all cells from SCROT and NSR donor groups. Bar indicates median. (C-D) Number of CD19+ B cells and CD19+CD27+IgD−CD38− memory B cells (MBCs) assessed via flow cytometry at baseline for patients with SCROT, SROT, and NSR. (E-F) Percentages of CD27+CD38int/+CD71+ activated B cells (ABCs) and CD27highCD38high antibody–secreting cells (ASCs) assessed via flow cytometry at baseline for patients with SCROT, SROT, and NSR. (G) Optical densities (ODs) of anti-GPIIbIIIa antibodies (Abs) measured using specific immobilization of platelet antigen assay at baseline for patients with SCROT, SROT, and NSR. (H-K) Percentages of CD3+CD4+CCR7−CD45RA− memory (TEM), CD3+CD4+CCR7+CD45RA− central memory (TCM), CD3+CD4+CXCR5+ICOS+PD-1+ circulating T-follicular helper (cTFH), and CD3+CD4+CD25highCD127low Treg CD4+ at baseline for patients with SCROT, SROT, and NSR. (L) Frequency of GPIIbIIIa-specific CD4+ T cells assessed via flow cytometry, with an adapted antigen-reactive T-cell enrichment technology at baseline for patients with SCROT (platelet count >100 × 109/L, SROT [platelet count 30 × 109/L to 100 × 109/L], and NSR [platelet count <30 × 109/L and or bleeding]). Bars indicate median. Kruskal-Wallis and Wilcoxon-Mann-Whitney tests were used as appropriate. Mono, monocyte; NK, natural killer; pDCs, plasmacytoid dendritic cells; RBCs, red blood cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/23/10.1182_blood.2022018665/2/m_blood_bld-2022-018665r1-gr3.jpeg?Expires=1770868224&Signature=GT27sVng5XUz1YGgGc~k83V4gVbz1ypcK7~~x9lrNTvFuFoudGBk4CxzM8DBnyTr9CS0iFAjNhfOOM39tI3Hwv30sve0yF8T7wvyJP-lb~nMuG4HTcvNoLXE-ywlBcwWiFl~haWRy-dKYdS6Fkh-d1rnNGLugCCppUdaQT8Cjgxbzcdl0Vz4Ko0jy5xi9jrQkfq5APYSsFCBHveXYtJaWwtESkM5ImpC7jKtWhPhqt2NnvfYiCkojFclgd~qWnD9bNDqQa3mLCDQ6tZ0IBoPwGwL7epJ9pDNT-vwGCjbMCVsnNkpY7e7rsL858cZgj7u1Jr1rd1ddYqETxAqVYv6ZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Differential gene expression analysis identifies CD69 expression in CD8 T cells as a predictor of NSR. (A) Plot showing the preranked gene set enrichment analysis results for comparison of patients with SCROT vs those with NSR in lymphoid cells and CD14+ monocytes clusters. Dot size represents the normalized enrichment score (NES). Dot color represents the sign of the log fold change ∗ log10(false discovery rate [FDR] value) (ie, blue indicates enrichment in patients with SCROT and red indicates enrichment in patients with NSR). Only gene sets with NES >1.5 and FDR <0.05 in at least 1 of the comparisons are shown. (B) Violin plot of TNF-α signaling via NF-κB pathway signature in CD8+ memory T cells from patients with SCROT and NSR at baseline (left). Enrichment plots for TNF-α signaling via NF-κB gene set in the comparison of patients with SCROT vs those with NSR (right). (C) Dot plots showing expression of expressed genes from TNF-α signaling via NF-κB gene set in CD8+ memory T cells from patients with SCROT and NSR at baseline. Dot sizes represent the percentage of cluster cells in which transcripts for that gene are detected. Dot color represents the average expression level (scaled normalized counts) of that gene in the population. Percentages of CD3+CD8+CD69+ (D) or CD3+CD4+CD69+ (E) T cells assessed via flow cytometry after overnight resting at baseline for patients with SCROT, SROT, and NSR. Bars indicate median. The Kruskal-Wallis and Wilcoxon-Mann-Whitney tests were used as appropriate. Only significant P values are reported; ∗∗P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/23/10.1182_blood.2022018665/2/m_blood_bld-2022-018665r1-gr4.jpeg?Expires=1770868224&Signature=Q7S7NRGXH6T~oHDd2C~GwCWcr-SRhug5HECPfO0TsSmIobN4Wm6U3C3bU2RGpFDcmMXAE2VDLHwNuZH46ClMojrwH7Ku9obnOoXVQYiE7Reg3D44zB32gfcaFNiz3RuJ3pdbzT8dVi1sUxwzGTM9qWexeuwIVoUl7yN8MdPkNYW00xl6oXKFw9yzKEYjfeXzAoGeN4A1rYe2BxPXS5lUy2~pLMyeEG7n2zAHtKxThFOP9nt8gMnDYGlk7aOLfUN9~qQ9qzV6V7IMUtaIvZm~ruMzWGLXpLgwWeEwbK-kul9yAZ67m6aR7NSRFttZklAcEyYirlAKkg7~9GjwlayPJw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal