In this issue of Blood, Sukhbaatar et al identify an unexpected role of lamina propria macrophages as regulators of dietary iron absorption by controlling availability of the plasma iron acceptor transferrin.1
Dietary iron absorption is essential to maintain systemic iron balance. Although the underlying mechanism is fairly well understood, at least for inorganic iron, important regulatory aspects remain unexplored. Iron from digested food is assimilated in the duodenal lumen by enterocytes. Iron is then transferred to the basolateral site of these epithelial cells and released to the bloodstream via the ferrous exporter ferroportin. Following oxidation to the ferric form by ferroxidases such as hephaestin and ceruloplasmin, absorbed iron is finally captured by the plasma iron carrier transferrin for delivery to tissues. Dietary iron absorption is highly regulated to maintain sufficient supplies for metabolic needs and to prevent development of iron deficiency or overload states,2 which are associated with clinical complications. A major security check occurs during iron entry into plasma and involves regulatory networks that converge on ferroportin.3 Thus, ferroportin gene expression is subjected to transcriptional and posttranscriptional control, whereas protein levels and iron export activity are regulated by the iron hormone hepcidin.4
Sukhbaatar et al show that transferrin availability is another critical determinant of iron absorption. They utilized mice (cre/+) engineered for macrophage-specific ablation of tuberous sclerosis complex 2 (Tsc2), a suppressor of the nutrient sensor and metabolic regulator mTORC1. Unrestricted mTORC1 activity in macrophages of these animals is associated with systemic iron deficiency. This is manifested in hypochromic and microcytic erythrocytes, low transferrin saturation, reduced number of mature erythroid progenitors, splenomegaly, extramedullary erythropoiesis, and depletion of bone marrow and splenic iron stores. The iron deficiency phenotype of cre/+ mice could be largely corrected by pharmacological inhibition of mTORC1 with everolimus, indicating a causal relationship with TORC1 hyperactivity in macrophages.
Cre/+ mice retain excess iron within duodenal enterocytes, which suggests a primary defect in dietary iron absorption. In fact, iron deficiency was rescued by parenteral iron administration that bypasses the gut barrier. Notably, the apparent block in iron export from the duodenal epithelium to plasma occurred despite increased ferroportin levels in enterocytes and with physiological expression of hephaestin and ceruloplasmin. The hepcidin-encoding Hamp gene was appropriately suppressed in iron-deficient cre/+ mice, even though quite unexpectedly, circulating hepcidin levels were not reduced. Overall, these data point to a novel mechanism that hampers dietary iron absorption in cre/+ mice.
The key to unraveling this mechanism was the analysis of transferrin expression in duodenal sections. This revealed that transferrin was depleted in the villous interstitium of the lamina propria (LP) in cre/+ mice, contrary to wild-type control animals. The defect was restricted to the LP, as total duodenal transferrin content or transferrin expression in adjacent muscularis mucosa did not differ among genotypes. Proper transferrin localization in the LP of cre/+ mice could be restored with everolimus, again highlighting the link with mTORC1 activity in macrophages. Moreover, the correction of systemic iron deficiency by everolimus suggested that efficient iron efflux from the basolateral site of enterocytes to plasma requires presence of the iron acceptor transferrin in the LP.
Importantly, depletion of macrophages by a blocking antibody against colony-stimulating factor-1 receptor significantly enriched transferrin in the LP of wild-type mice. On the other hand, mTORC1 signaling was shown to induce the activation marker CD68 in LP macrophages, which inversely correlated with transferrin abundance. These findings reinforce the critical role of LP macrophages as negative regulators of local transferrin expression and, consequently, of dietary iron absorption. LP macrophages exhibit a unique immunological profile and are important contributors to intestinal immune and physiological responses.5 Consistent with this view, infection of wild-type mice with Citrobacter rodentium or overnight starvation of cre/+ mice promoted transferrin accumulation in the LP, presumably by mechanisms impairing mTORC1 signaling. Furthermore, refeeding resulted in clearance of LP transferrin in both wild-type and cre/+ mice.
Hints on the mechanism by which LP macrophages control local transferrin expression were provided by single cell transcriptomics analysis of CD45+ LP cells. This uncovered increased expression of several cathepsin proteases in CD68+-activated LP macrophages from cre/+ mice, as well as enrichment of lysosome and phagosome-related pathways. The data also showed upregulation of the macrophage-specific subunit Atp6v0d2 of vacuolar H+-ATPase, an enzyme that activates lysosomal digestive proteases in endosomes via acidification. In line with these observations, cre/+ LP macrophages had increased capacity to engulf transferrin ex vivo and possessed higher protease activity in situ. Moreover, the serine protease inhibitor nafamostat could rescue transferrin localization in the LP of cre/+ mice, promoting a significant increase in plasma transferrin saturation.
Taken together, the data provide strong evidence that mTORC1-activated LP macrophages limit local expression of transferrin via proteolytic degradation, resulting in decreased efflux of dietary iron from enterocytes to plasma (see figure). The mechanism is regulated during the prandial process and in response to infection. Thus, LP macrophages and transferrin emerge as new players in the control of dietary iron absorption. Understanding their exact function is important and has a translational potential, as iron deficiency affects almost one-quarter of the world population.6
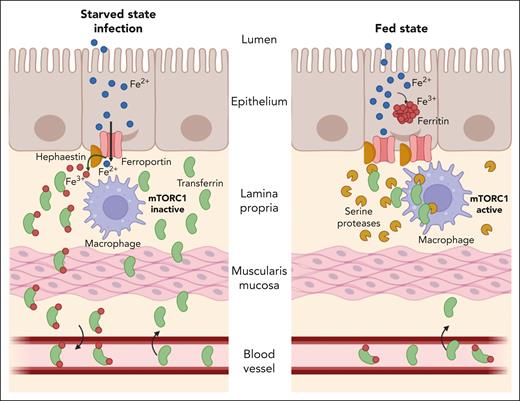
A model for regulation of dietary iron absorption by LP macrophages. During the starved state or following infection, the mTORC1 pathway remains inactive in LP macrophages (left). This allows proper localization of transferrin in the LP interstitium, which in turn promotes iron efflux from enterocytes via ferroportin. Activation of mTORC1 signaling in LP macrophages during the fed state induces expression of serine proteases that target transferrin for degradation (right). Absence of the iron (Fe) acceptor from the LP impairs iron efflux from enterocytes, despite high expression of ferroportin, and promotes its sequestration within ferritin.
A model for regulation of dietary iron absorption by LP macrophages. During the starved state or following infection, the mTORC1 pathway remains inactive in LP macrophages (left). This allows proper localization of transferrin in the LP interstitium, which in turn promotes iron efflux from enterocytes via ferroportin. Activation of mTORC1 signaling in LP macrophages during the fed state induces expression of serine proteases that target transferrin for degradation (right). Absence of the iron (Fe) acceptor from the LP impairs iron efflux from enterocytes, despite high expression of ferroportin, and promotes its sequestration within ferritin.
Although the study by Sukhbaatar et al offers new insights on iron absorption, it also raises several mechanistic and pathophysiological questions. For instance, does transferrin present in the LP originate from the circulation, or is it, at least in part, locally produced? Does transferrin clearance by LP macrophages involve its uptake via transferrin receptor 1 (Tfr1)? In light of the low binding affinity of apo-transferrin to Tfr1,7 is an alternative mechanism possible? Is the transferrin-degrading capacity restricted to LP macrophages and, if yes, how is specificity maintained? Can the large apo-transferrin reservoir in plasma be mobilized to increase transferrin localization in the LP and reverse the inhibitory effects of LP macrophages and, if yes, under which conditions? Why is local degradation of transferrin in the LP detrimental for iron absorption, considering that patients and mouse models with either dramatically reduced or almost fully saturated transferrin due to hereditary hypotransferrinemia or hemochromatosis, respectively, efficiently assimilate dietary iron?
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal