In this issue of Blood, Socié et al1 reported the prognostic value of 21 blood biomarkers in predicting the treatment response of steroid-refractory or steroid-dependent acute graft-versus-host disease (GVHD) using samples collected in the phase 3 REACH2 trial (ruxolitinib vs best available treatment; NCT02913261).2 The authors identified several baseline clinical factors and biomarkers at baseline and at day 14 after treatment that predicted probabilities of treatment response.
Acute GVHD remains a major limitation of allogeneic hematopoietic cell transplantation. Systemic, high-dose glucocorticoids are the recommended first-line treatment for patients with more than mild acute GVHD. The goal of therapy is to control the acute manifestations of GVHD with its potential for causing life-threatening organ damage and to taper the dose of glucocorticoids as soon as clinically possible. Approximately 60% of patients respond to initial treatment with glucocorticoids,3,4 but the remaining patients (with either steroid-refractory or steroid-dependent GVHD) require second-line systemic treatment. No clearly superior second-line systemic treatment for steroid-refractory or steroid-dependent acute GVHD has been identified, despite many clinical trials. The recently reported REACH2 randomized trial showed that ruxolitinib was more effective than the investigator’s best choice of other therapies.2 The overall response rates at day 28 were 62% with ruxolitinib and 39% with best available therapies. However, we still do not know which patients are more likely to respond to second-line systemic treatment. Identification of biomarkers and clinical factors that predict treatment response to the second-line systemic treatment would further guide treatment decisions.
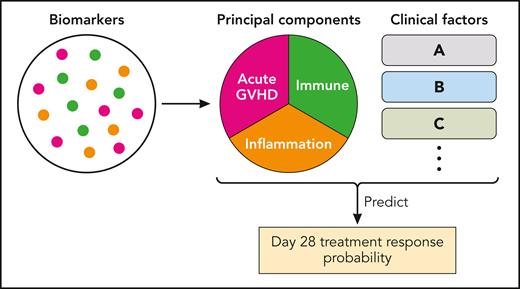
Using samples collected in the REACH2 trial, Socié et al succeeded in identifying biomarker profiles and clinical factors that predict treatment response at day 28 after second-line systemic treatment. A total of 295 patients at baseline, and 242 patients at day 14 after second-line systemic treatment, were included in the analysis, with half of the patients treated with ruxolitinib and the remaining patients treated with best available therapies. A total of 29 biomarkers (proteins or immune cell subsets) were measured, and 8 biomarkers were excluded because baseline values exceeded limitations of quantitation or because of lack of change over time, leaving 21 biomarkers for the final analysis. Notably, the authors generated acute GVHD-, immune-, and inflammatory-related principal components to account for complex interactions between multiple biomarkers, and the principal components were used as variables together with other important clinical variables (see figure). Important to note is that these principal components were determined without referring to the data on treatment response. The authors found that treatment with ruxolitinib, skin involvement, reduced-intensity conditioning, and younger patient age were associated with treatment response. Lower levels of several biomarker-derived immune or GVHD-related principal components both at baseline and at day 14 were associated with treatment response. A notable finding is that change in biomarkers from baseline over time was not useful. Treatment with ruxolitinib was the strongest factor associated with treatment response. The bias-corrected areas under receiver operating characteristic values were reasonably high (0.73 for the baseline model and 0.80 for the day-14 model), showing the potential of this approach and the need for continued refinement.
The analytic method used by Socié et al. The biomarkers were grouped into 3 major categories of principal components and then analyzed with clinical factors. GVHD, graft-versus-host disease. Professional illustration by Patrick Lane, ScEYEnce Studios.
The analytic method used by Socié et al. The biomarkers were grouped into 3 major categories of principal components and then analyzed with clinical factors. GVHD, graft-versus-host disease. Professional illustration by Patrick Lane, ScEYEnce Studios.
The Mount Sinai Acute GVHD International Consortium (MAGIC) biomarkers (ST2 and REG3α) have been validated in several studies of initial systemic treatment for acute GVHD.5 A notable point is that both proteins showed reasonably large differences between responders and nonresponders in this study. Also interesting is that the B cell marker and IL-6 showed the largest differences between the groups, although B cells are thought to be conventionally more important in chronic GVHD than in acute GVHD. The biological implications of these observations need further study. As acknowledged by the authors, the biomarker panel could be optimized further, without losing much precision, by selecting biomarkers with large principal component coefficients. Panels with fewer biomarkers would be more realistic for routine clinical use.
What were the most relevant biomarkers for predicting treatment response specifically to ruxolitinib? The authors explored interaction analyses and found no differential response in biomarker subgroups between the treatment arms. Thus, the current models predict treatment response regardless of treatment type, and further studies are warranted to identify biomarkers that predict treatment response specifically to ruxolitinib. An important goal now is to define ruxolitinib-refractory or -dependent patients with GVHD. A new working definition was proposed recently, with at least 14 days of treatment recommended to define lack of improvement.6
How will the models reported here inform practice? An important point to recognize is that this approach is a probability engine that tells us probabilities of response, rather than a classification engine that tells us positive and negative predictive values in predicting response. By using the probability engine, we will know an expected probability of response in an individual patient with steroid-refractory or steroid-dependent acute GVHD. Such information may help inform our clinical decision when we need to start second-line systemic treatment. Furthermore, if we can predict the probability of subsequent response based on clinical and biomarker information at day 14 or even earlier, this prediction may help us in initiating third-line treatment earlier. Future studies should clarify reliable cutoff probabilities for treatment choice or change. Verification is needed in independent cohorts to prove the applicability and utility of the current models for use in clinical trials and in practice.
Conflict-of-interest disclosure: Y.I. received honoraria for speaker fees from Janssen, Meiji Seika Pharma, and Novartis, and research funding from Amgen.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal