In this issue of Blood, Odak et al1 investigate locations in the noncoding genome that provide a stable expression of CAR transgenes in human T cells. This study expands our ability to engineer potent CAR T-cell products and provides resources for the identification of other extragenic genomic safe harbor locations.
Chimeric antigen receptor (CAR) T cells are biologic drugs genetically reprogrammed to recognize and kill cancer cells expressing the target antigen. Therefore, a prerequisite for effective CAR T-cell therapy is the long-term expression of the CAR at an optimal level in the T cells, thereby creating a drug that can have multiple reengagements with cancer cells.
Conventional gene transfer using retroviral vectors and transposons results in a semirandom integration of CAR expression cassettes with varying copy numbers. This process may lead to the disruption of endogenous gene expression, yielding genetically heterogenous cell products with uneven clinical potency. CAR expression levels in individual T cells may also differ, reflecting the variation in transgene copies per cell, positional effects of the integrated provirus, and the epigenetic silencing of exogenous DNA. Furthermore, excessively high CAR expression levels can produce toxicity in T cells and accelerate their differentiation and dysfunction.2
Targeted integration of CAR transgenes in defined genetic locations can help overcome these challenges and reduce the risks associated with conventional gene transfer methods, such as insertional mutagenesis. Previously, Eyquem et al3 demonstrated T cells expressing the CAR transgene from the T-cell antigen receptor (TCR) α chain locus, which was generated using CRISPR-Cas9, outperformed retrovirally transduced CAR T cells in xenograft models. However, the resulting disruption of the TCR expression may not always be desirable4-6 (especially for autologous products), and the level of expression may not be optimal for all CARs. The consequences of this homology-directed repair–mediated gene insertion into a transcriptionally active region of chromatin and its effect on the regulation of surrounding genes is not well understood. These considerations led the authors to identify extragenic genomic safe harbors (eGSHs) in the noncoding regions of T-cell chromatin that are suitable for long-term “CAR parking” and do not interfere with the functional genome architecture, thus improving the safety profile.
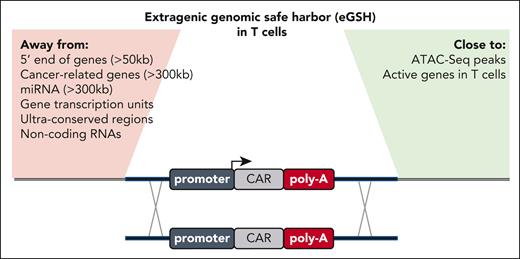
For the effective integration of large genetic sequences, such as CAR transgenes, Odak et al used a CRISPR-Cas9–assisted knockin technique previously developed for T cells by the same group.3 Transgene integration is facilitated by homologous DNA repair after transfection of the CRISPR-Cas9 programmable nuclease and delivery of a DNA donor template encoded via a nonintegrating adeno-associated virus serotype 6 viral vector.3 To predict the potential eGSHs, the authors used their previously established rules,7 such as ensuring that there is sufficient distance from protein-coding genes related to cancer and noncoding RNAs (see figure). Because of its vast size, the noncoding genome of our DNA provides too many possible locations for empirical testing via gene editing. Therefore, the authors hypothesized that the optimal eGSHs should be sequences within open chromatin that would (1) be targetable with conventional programmable nucleases and (2) allow for effective and durable transgene expression with transgenic expression cassettes.
Overview of the expanded list of criteria to identify a good eGSH locus in T cells. Previously proposed rules are highlighted on the left (Away from), and newly added criteria by Odak et al are listed on the right (Close to).
Overview of the expanded list of criteria to identify a good eGSH locus in T cells. Previously proposed rules are highlighted on the left (Away from), and newly added criteria by Odak et al are listed on the right (Close to).
To test their hypotheses, the authors mapped accessible chromatin regions in the activated human CD4+ and CD8+ T cells and generated a list of locations accessible in both cell types. Subsequently, they demonstrated that the CRISPR-Cas9 cleavage was most efficient for close to open regions identified by the presence of ATAC-seq peaks, which is in line with previous reports about other mammalian cell types. Next, they designed knockin templates to introduce CAR transgene expression cassettes into the identified eGSHs in human T cells. After CAR knockin, all 6 eGSH locations enabled CAR expression, albeit at variable levels and durations. All eGSH-driven CAR T cells produced the expected dose-dependent cytolysis of tumor cells. However, despite the use of the strong mammalian EF1a promoter, in 5 out of 6 of the initially selected eGSHs, CAR expression was gradually lost during T-cell expansion. Only eGSH6 promoted long-term CAR expression that translated to effective tumor control in vitro and in several in vivo xenograft mice models designed to stress-test the eGSH6-integrated CAR T cells.
The mechanisms underlying the rapid CAR silencing from most eGSHs were explored, but some remain elusive. Because eGSH6 was in proximity of a pseudogene, 4 additional eGSHs with neighboring pseudogenes were tested with discouraging results: all but eGSH6 were silenced after the first CAR engagement. To test their newly expanded set of criteria (see figure) for the identification of eGSH, the authors reanalyzed data from retrovirally transduced–hematopoietic stem cell products and tracked data from patients with severe combined immunodeficiency (SCID). They argued that random integration could happen both in functional and nonfunctional eGSHs. Functional eGSH locations would allow for the differentiation of T cells in patients with SCID because of the active expression of the gene therapy cargo, whereas nonfunctional eGSHs would be lost. Their elegant approach supports the notion that in addition to open chromatin, functional eGSH may lie adjacent to active genomic regions in T cells.
T-cell engineering via the eGSH6 locus identified in this study may be useful for diverse clinical applications. The expression cassettes with or without genetic insulator elements had only minimal impact on the surrounding genes or the closest noncoding RNA. Beyond fostering constitutively expressed transgenes, such as CARs or cytokines, eGSH6 can be explored to harbor complex transgenes, including inducible promoter systems, thus enabling enhanced T-cell products by synthetic regulatory circuits, such as SynNotch and SNIPR.8 The utility of eGSH6 for gene therapies for T-cell–related immunodeficiencies can also be investigated.
Odak et al have successfully identified 1 eGSH permissive for a durable and predictable level of transgene expression. The high rate of failure (10 eGSHs were identified and tested using a refined set of rules) shows our limited understanding of the noncoding genome. Misinterpreted as “junk” DNA for many decades, we have just begun to decipher the roles of noncoding DNA in cell fate and development.9 The nonfunctional eGSH identified by Odak et al may be useful in exploring the regulatory mechanisms that restrict transgene expression in the noncoding genome of T cells. When sufficiently understood and combined with new gene editing strategies, such as those linking the programmability of CRISPR-Cas with the cargo capacity of integrases,10 targeted transgene integration can be used to build safe and robust synthetic genetic architectures for therapeutic purposes.
Conflict-of-interest disclosure: D.L.W. is an inventor on multiple patent applications related to cell and gene therapy and a scientific cofounder of TCBalance Biopharmaceuticals GmbH. M.M. is an inventor on multiple patents and patent applications related to engineered T cells; received licensing fees and payments from Fate Therapeutics, Allogene Therapeutics, and March Biosciences and honoraria/sponsored travel from Amgen and Fate Therapeutics; has a sponsored research agreement with Fate Therapeutics; receives consulting fees from Xenetic Biosciences and March Biosciences; and has equity in March Biosciences.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal