In this issue of Blood, Jajosky et al1 utilize murine models of red blood cell (RBC) alloimmunization to show that intracellular polymorphisms can facilitate alloantibody responses to unrelated surface antigens and may induce responses in otherwise nonresponsive animals. Priming to cytosolic antigens may contribute to the hyperresponsiveness to additional RBC surface alloantigens seen in some repeatedly transfused patients, despite the transfusion of blood that is crossmatch compatible for all known alloantibodies.
RBC transfusion and pregnancy provide the most common sources of exposure to tissue alloantigens in clinical practice and in nature, respectively. The potentially fatal outcomes of RBC alloimmunization, including hemolytic transfusion reactions and hemolytic disease of the fetus and newborn (HDFN) (erythroblastosis fetalis), are minimized but not eliminated by the widespread adoption of empirically derived approaches: RBC compatibility testing and provision of antigen-negative blood to prevent hemolytic transfusion reactions; and the use of leukoreduced blood products or the passive administration of Rh immune globulin to prevent alloimmunization. Despite many scientific advances that include the recognition of 43 polymorphic RBC surface antigen systems containing 349 red cell antigens determined by 48 genes (many first identified using clinical alloantibodies),2 there is a poor understanding why most patients never make RBC alloantibodies even if frequently exposed to genetically diverse RBC transfusions in chronic transfusion protocols (eg, in sickle cell anemia or thalassemia).3 Clinical science is unable to predict who will respond (responders) and who will not respond (nonresponders) to a given or even to any RBC alloantigen. Furthermore, we do not fully understand why certain RBC antigenic polymorphisms are more immunogenic than others or why some patients who make one alloantibody are much more likely to make additional alloantibodies to other RBC antigens (hyperresponders),4 making sourcing of compatible blood difficult and sometimes impossible.3 Transfusion recommendations for chronically transfused patients with sickle cell anemia recommend providing RBCs compatible for a limited set of ABO, Rh (C/c, D, and E/e), and Kell antigens until a patient has been shown to respond to any antigen (ie, to emerge as a responder phenotype) before recommending the use of extended phenotype (Jka/Jkb, Fya/Fyb, M/N, and S/s) matching to prevent additional alloimmunization.5 This approach saves on resources and expense, but exposes patients to a level of risk. A better understanding of the nonresponder phenotype may help identify responders before they are alloimunized, permitting the use of extended phenotype matching to prevent the first alloantibody.6 It may also provide insights into the nature of immune tolerance and even suggest therapeutic approaches to revert responders into nonresponders after alloimmunization.
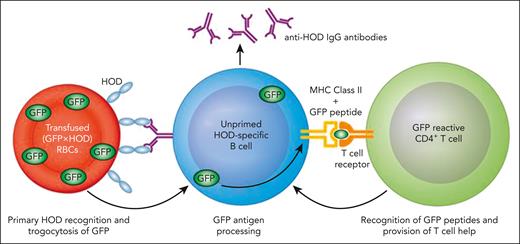
It would be unethical to deliberately expose volunteers to allogeneic RBCs for the sole purpose of trying to induce alloimmunization. To this end, Jajosky et al use an elegant set of transgenic murine models and flow cytometry tools.7 Green fluorescent protein (GFP) provides an RBC cytoplasmic antigen, whereas transgenes expressing chimeric hen egg lysozyme (HEL), ovalbumin, and human Duffy b (HOD); or human glycophorin A (GPA) display antigenic epitopes on the RBC cell surface.7 The authors have previously shown that Kell-positive RBC transfusion in the presence of inflammation not only enhances anti-Kell antibody production but also directly facilitates anti-HOD antibody formation following subsequent exposure to the disparate HOD antigen, demonstrating that immune priming to one surface RBC alloantigen can directly enhance a humoral response to a completely different surface RBC alloantigen.4 These data suggested that B cells possess the capacity to process 2 distinct RBC cell surface antigens, with T-cell help derived from one protein facilitating antibody production to the other. However, as HOD and Kell are on the same RBC surface, and RBC alloantigens can be linked in large macromolecular complexes, it remained possible that this simply represents the “linked recognition” that forms that basis of conjugate vaccine design. As a result, whether a completely distinct antigen not physically associated with the target antigen can prime a recipient to an unrelated surface alloantigen remained unknown.
Jajosky et al demonstrate that prior priming to an unlinked cytosolic protein, GFP, can enhance alloimmunization to completely unrelated surface alloantigens. Initial experiments show that B cells from IghelMD4 transgenic mice that produce IgG specific for HEL preferentially internalize GFP from (GFP × HOD) RBCs that express the HEL antigen on their RBC surface, suggesting a trogocytosis process (internalization of RBC contents, including cytosolic proteins during antigen recognition) (see figure). In a subsequent key experiment, they show that mice primed to the GFP protein preferentially produce antibodies to HOD epitopes, only if immunized with (GFP × HOD) splenocytes, suggesting that T-cell help specific for cytoplasmic GFP epitopes can augment the response to the cell surface HOD antigen. This finding flies in face of conventional dogma that B cells present antigens when a cognate B-cell receptor recognizes a protein epitope on the same (or linked to a) protein as its corresponding CD4 T-cell epitope. Enhanced anti-HOD responses were shown to not be due to augmentation by anti-GFP antibodies or due to increased numbers of antigen-specific B cells. More important, these experiments were repeated using RBC cell surface human GPA as an antigen. Mice that were usually nonresponders to GPA in the absence of inflammation could be converted to respond when primed with intracellular GFP and presented with GPA in the context of (GPA × GFP) RBCs. These data provide a plausible explanation for the hyperresponsiveness to RBC alloantigens seen in some transfusion recipients. Intracellular polymorphisms are not assessed in the routine clinical crossmatch compatibility process, and T cells primed to those antigens may augment subsequent antibody responses. It is not currently feasible to provide RBCs that are compatible for intracellular antigens.
Proposed model for enhancement of RBC alloimmunization by prior immunization to an unrelated intracellular antigen. MHC, major histocompatibility complex. Professional illustration by Patrick Lane, ScEYEnce Studios.
Proposed model for enhancement of RBC alloimmunization by prior immunization to an unrelated intracellular antigen. MHC, major histocompatibility complex. Professional illustration by Patrick Lane, ScEYEnce Studios.
Prior work in similar murine models has shown that the responder status is under the control of regulatory T cells8; that tolerance can be induced to RBC alloantigens9,10; and that tolerogenic signals can be converted to stimulatory signals under defined circumstances.9,10 These relatively simple murine models for understanding immunity and tolerance to tissue alloantigens are providing powerful insights that ultimately may find application in clinical organ transplantation and the treatment of autoimmunity, as well as in treating HDFN and facilitating transfusion without the risk of hemolysis.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal