In this issue of Blood, Biondi et al1 show that enforcing CXCR4 expression on CD33-targeting chimeric antigen receptor (CAR) cytokine-induced killer (CIK) cells enhances their homing to the bone marrow (BM) and improves their activity against acute myeloid leukemia (AML).
CXCR4 is a chemokine receptor that binds to CXCL12, also known as SDF-1, which is produced by BM stromal cells. Binding of CXCR4 to CXCL12 plays an important role in retention of hematopoietic stem and progenitor cells (HSPCs) in the BM. This is most evident by the use of plerixafor, a CXCR4 receptor antagonist, in clinical practice for HSPC mobilization.
AML is a malignancy that is derived from HSPCs, and CXCR4 expression is also observed on AML and is associated with poor prognosis.2 One can presume that the mechanisms used to support HSPCs in their BM niche can also support AML, particularly the leukemic stem cells (LSCs) that serve as a nidus for disease relapse. Therefore, expressing CXCR4 on immune effector cells can bring them into close proximity with LSCs and increase the probability of achieving durable remissions in AML.
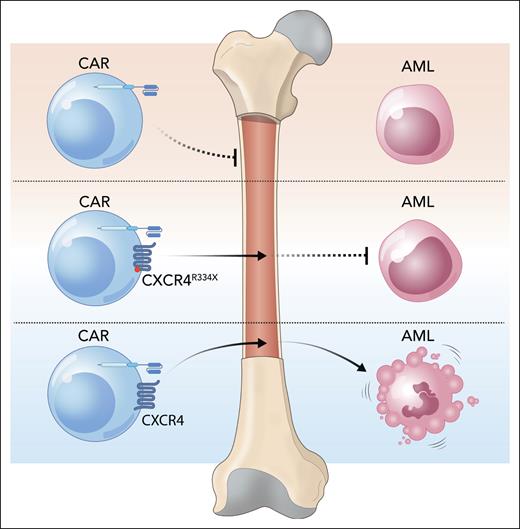
Initially, the authors examined CXCR4 expression on CD33-targeting CAR-CIK cells. Of note, the CIK cells used by the authors refer to a particular composition of effector lymphocytes that harbor a mixed T and natural killer (NK) cell phenotype and so are somewhat different from the T cells or NK cells that are commonly used for adoptive cell therapy.3 The authors show that CD33.CAR-CIK cells have reduced expression of CXCR4 compared with normal T cells and so may be impaired in their ability to migrate to the BM.
To address this concern, the authors investigated coexpressing CXCR4 with the CAR, either in its native format or a mutant CXCR4R334X. The mutant CXCR4R334X was discovered in patients with warts, hypogammaglobulinemia, recurrent bacterial infection, and myelokathexis (WHIM) syndrome and has been shown to increase HSPC retention in the BM.4 The authors hypothesized that the mutant CXCR4 would further improve BM homing of CIK cells and thus their antileukemic effect.
The first part of this hypothesis proved to be correct. CD33.CAR-CIK cells expressing native CXCR4 showed increased migration toward CXCL12 in vitro, which was further increased with expression of mutant CXCR4. Additionally, the authors took advantage of the cross-reactivity of the human CXCR4 receptor toward murine CXCL12 to show that native CXCR4-expressing human CD33.CAR-CIK cells have enhanced homing into mouse BM in vivo, and this is again more pronounced with the mutant CXCR4.
However, the mutant CXCR4 receptor, despite its superior BM homing capabilities, did not enhance CAR-CIK cell antileukemic activity (see figure). In mice engrafted with KG-1, a CD33+ AML cell line, only CD33.CAR-CIK cells expressing the native CXCR4 led to better clearance of leukemia and survival. This suggests that the presence of native CXCR4 not only affects CAR-CIK cell homing but also enhances their cytolytic function, and it is the latter that is more important for disease clearance in this model. Indeed CAR-CIK cells with the native CXCR4, but not the mutant, showed improved tumor-cell killing in vitro. This seems to be due to the ability of the native CXCR4 to stabilize the immunological synapse, which is lacking in the mutant CXCR4.
CXCR4 helps CAR-CIK cells reach the BM and eliminate AML. Adding CXCR4, a chemokine receptor, to CAR-CIK cells increases their homing to the BM and also improves cytotoxic activity against AML. Mutant CXCR4R334X improves BM homing but does not increase CAR-CIK cell cytotoxicity. Professional illustration by Somersault18:24.
CXCR4 helps CAR-CIK cells reach the BM and eliminate AML. Adding CXCR4, a chemokine receptor, to CAR-CIK cells increases their homing to the BM and also improves cytotoxic activity against AML. Mutant CXCR4R334X improves BM homing but does not increase CAR-CIK cell cytotoxicity. Professional illustration by Somersault18:24.
It will be interesting to see whether these findings are broadly applicable to different types of immune effector cells or are limited to the specific CIK cells used in this study. The initial premise of using CXCR4 to target LSCs also remains to be validated, as the model used in this study was not designed to assess the activity of CAR-CIK cells against LSCs. Nevertheless, the authors’ serendipitous finding uncovers an added and unexpected benefit to using CXCR4 as a therapeutic tool that may enhance T cell immunotherapy.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal