In this issue of Blood, Diamond et al reconstruct clonal trajectories that lead to therapy-related myeloid neoplasms (tMNs), showing emergence from preexisting clones that either harbor chemotherapy mutation signatures or escaped exposure via apheresis.1
In spite of the enormous benefits of chemotherapy, it can plant the seed for the development of a secondary neoplasm years or decades later. Although the existence of tMNs was recognized decades ago, the genomic events that give rise to clonal expansion and transformation remain poorly defined.2,3 An unresolved question is whether chemotherapy directly induces the acquisition of new driver mutations or whether it selects for existing preleukemic clones. To answer these and other questions, Diamond et al use mutation signatures of platinum- and melphalan-containing chemotherapies, and leverage genetic aberrations as temporal barcodes. In so doing, they provide evidence for 2 common evolutionary trajectories from primary disease to secondary malignancy.
The authors first assembled a dataset of whole-genome sequencing (WGS) on a cohort of 40 tMNs with primary malignancies across solid tumors, multiple myeloma (MM), and non-Hodgkin lymphoma (NHL). They further imported tMN and de novo acute myeloid leukemia (AML) data from public datasets such as the Cancer Genome Atlas (TCGA) and Beat AML.4-7 The authors quantified mutational processes, starting with single-base substitutions (SBSs), including the following: SBS1 and SBS–HSC related to age; SBS31 and SBS35 related to platinum-based chemotherapy; and SBS–MM1 caused by melphalan. The SBS mutation burden was significantly higher in platinum- and melphalan-exposed tMNs, compared to that in unexposed tMNs and de novo AML. All tMNs exposed to prior platinum-based therapy harbored the platinum signature, consistent with a model in which one cell survived platinum-based DNA damage and expanded to clonal dominance. Conversely, only 41% of tMNs exposed to prior melphalan-based therapy harbored the melphalan signature, consistent with the secondary malignancy being driven by subclonal populations, lack of melphalan-driven mutagenesis, or escape from melphalan exposure.
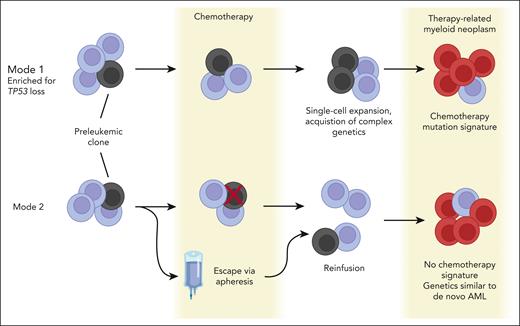
Building on these data, the authors describe 2 modes by which tMNs can emerge (see figure and next 2 paragraphs). Interesting to note is that both modes involve the selection of a preexisting clone by chemotherapy. By intersecting postplatinum tMN WGS data with clonal hematopoiesis (CH) mutations from a large patient cohort treated with platinum-based therapies,8 the authors find only a minor platinum contribution to nonsynonymous mutations in prevalent driver genes. Thus, rather than directly causing driver mutation acquisition, chemotherapy exposure selects for driver mutations.
Two modes of therapy-related myeloid neoplasm (tMN) evolution. Figure created with Biorender.com. AML, acute myeloid leukemia.
Two modes of therapy-related myeloid neoplasm (tMN) evolution. Figure created with Biorender.com. AML, acute myeloid leukemia.
In the first mode of tMN development, chemotherapy selects for a CH clone by creating an environment that is permissive to the clonal expansion of a mutated precursor. Chemotherapy also facilitates the subsequent acquisition of complex genomic drivers. Compared to de novo AML and tMNs without the chemotherapy mutation signature, tMNs with chemotherapy-related signatures were enriched for copy number aberrations (CNAs), structural variants, templated insertions, chromoplexy, and chromothripsis. In 5 of 8 tMNs with chromothripsis, the SMARCA4 gene was amplified. Overexpression of SMARCA4 in Ba/F3 cells supports the possibility that it plays a role in leukemia cell proliferation and cytokine independence.
In the second mode of tMN development, the preexisting CH clone escapes chemotherapy exposure via apheresis and is reintroduced by autologous stem cell transplantation (ASCT). Although difficult to prove directly, this “escape” model is supported by 4 lines of evidence, as follows: the latency between chemotherapy exposure and tMN development did not influence penetrance of mutation signatures; patients sequentially treated with platinum and then melphalan/ASCT harbored only the platinum mutation signature; postmelphalan/ASCT B-ALL samples did not harbor the melphalan signature; and tumors that did not have a route of escape from melphalan did harbor its signature. Furthermore, targeted sequencing of pre-melphalan blood cells or apheresis samples revealed preleukemic mutations in 8 of 11 patients, including one case with an antecedent TP53-mutated CH clone in the apheresis product.
One of the most innovative aspects of the study is the use of mutation signatures as temporal barcodes that permit dating of chromosomal gains relative to a discrete mutagenic exposure in a patient’s lifetime. If gain of a chemotherapy-related mutation occurs along with a chromosomal gain, then the chemotherapy exposure preceded the chromosomal gain and vice versa. In all 8 tMN cases with chemotherapy signatures (first mode) that were amenable to temporal barcoding using this method, melphalan or platinum signatures were found within chromosomal gains, demonstrating that chemotherapy exposure preceded large CNAs. In addition to the prevalence of CNAs in tMNs with chemotherapy signatures, the authors found a significant enrichment of TP53 mutations, compared to tMNs without chemotherapy signatures. This finding suggests that TP53 loss may protect clonal precursor cells against chemotherapy exposure.
The authors extended temporal barcoding to 2 postplatinum MM cases, which are known to acquire chromosomal gains early.9 They used the age-associated SBS5 mutation signature to time events leading up to MM. The authors inferred that multichromosomal gains emerged in the second decade of life and that a most recent common ancestor cell emerged prior to the primary malignancy (ie, solid tumor) and the associated platinum exposure. Although the number of patients analyzed here is limited, these observations support the use of mutation signatures to time genetic events and reconstruct clonal dynamics of blood cells.
In conclusion, Diamond et al decipher how chemotherapy can serve as a double-edged sword. Although the sparsity of direct chemotherapy-induced driver mutations may be a relief, chemotherapy exposure selects for TP53-mutated CH and facilitates subsequent clonal evolution. A worthwhile future undertaking is to clarify mechanisms by which chemotherapy facilitates later acquisition of complex genetics. Furthermore, the length of time over which evolutionary trajectories can be traced warrants deeper investigation into the clinical utility of monitoring genetic lesions to predict the risk of progression to disease. Finally, this work demonstrates the impact that can be achieved by pairing clinical insight with computational genomics.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal