In this issue of Blood, Calabria et al show that intrathymic (IT) delivery of adeno-associated virus (AAV) vectors results in a site-specific integration within T-cell receptor (TCR) genes close to DNA breaks created by recombination-activating gene (RAG) enzymes during the variable, diversity, and joining [V(D)J] recombination.1
With tremendous advances in molecular diagnostic techniques, an increasing number of causative gene defects have been discovered in human diseases. Gene therapy (GT), which aims to fix gene defects, promises a potential cure for these diseases. AAV vectors are the leading platforms for in vivo GT. They have a high transduction efficiency, a broad range of target tissues, and a good safety profile. Because AAV vectors present predominantly episomal, they are not useful in treating highly proliferative cells such as hematopoietic and lymphoid cells owing to the dilution effect.2,3
Pouzolles et al injected AAV-ZAP-70 into the thymi of ZAP-70-deficient mice and found that IT injection of AAV-ZAP-70 transduced up to 5% of thymocytes, resulting in the rapid development of functional T lymphocytes and rapid reconstitution of the thymic medulla (within 10 days). The gene-corrected T lymphocytes persisted in the periphery for more than 40 weeks after treatment.4 Genomic integration was detected in the treated mice. In this issue of Blood, the same research team published the results of their study on the AAV integration profile in lymph nodes, spleens, livers, and brains of ZAP-70-deficient mice treated with IT AAV-ZAP70 injection.1 This study also included some wild-type mice with AAV-GFP IT injection and treated MeCP2 deficiency mice. They found that more than 90% of integrations in T lymphocytes were clustered within the TCR α, β, and γ genes. The insertion sites were mapped to DNA breaks created by enzymatic activity of RAGs during V(D)J recombination. In contrast, AAV integrations in the liver and brain were distributed across the entire mouse genome.1 This characteristic integration profile is likely related to the tendency of AAV vectors to integrate at DNA breakage sites,5 which may not occur with other types of vectors.6
These findings demonstrate that IT AAV delivery can take advantage of the physiological DNA breaks generated during TCR V(D)J gene recombination, overcome the limitation of AAV vectors, deliver therapeutic transgenes into the genome of T lymphocytes, and achieve a long-term therapeutic outcome (see figure). If the integration interferes with TCR gene rearrangement, transduced thymocytes with no functional TCR will most likely die instead of becoming malignant. Therefore, insertional mutagenesis and genotoxicity are not likely to be an issue in this gene delivery approach. Because transduced thymocytes go through the selection process in the thymus and may develop tolerance to the antigens of the AAV capsid and transgene, immunogenicity seems less likely to be a problem. IT AAV gene delivery may be particularly useful for treating inborn errors of immunity (IEIs) with a gene defect predominantly affecting T lymphocytes. It also provides an ideal method for generating in vivo chimeric antigen receptor (CAR) T cells to treat cancer. Because of the progressive involution of the thymus after birth, this IT GT strategy may not be effective for older children and adults with thymic atrophy. Since a similar gene rearrangement process occurs during B-cell development in the bone marrow (BM), similar integration may occur in the immunoglobulin genes of B lymphocytes if AAV vectors are injected into the BM. It would be interesting to examine peripheral B lymphocytes from animal models treated with an intra-BM injection of an AAV construct.
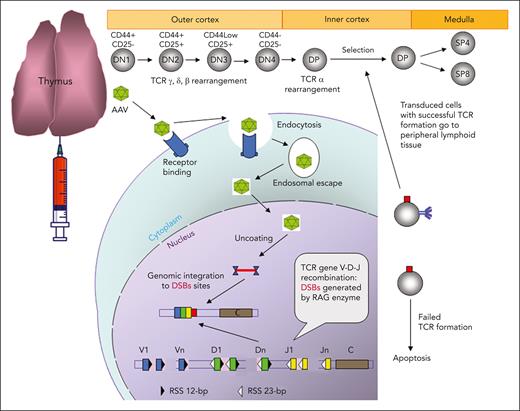
Schematic demonstration of IT AAV gene delivery. In the cortex of a thymus, thymocytes develop from CD4/CD8 double-negative 1 (DN1, early T-cell precursor) to CD4/CD8 double-positive (DP) stage, and these immature thymocytes have double-strand breaks (DSBs) created by RAG enzymes in TCR genes during TCR V(D)J recombination. AAV vectors enter thymocytes through receptor-mediated endocytosis, and the released AAV and transgene DNA in the nucleus is integrated into TCR genes close to the DSBs. The thymocytes with unsuccessful TCR formation owing to the AAV transgene integration will die. The transduced thymocytes with a functional TCR will undergo the selection process, and the positively selected thymocytes will develop into CD4 or CD8 single-positive (SP4 or SP8) T cells in the medulla and eventually be released into the peripheral lymphoid tissue.
Schematic demonstration of IT AAV gene delivery. In the cortex of a thymus, thymocytes develop from CD4/CD8 double-negative 1 (DN1, early T-cell precursor) to CD4/CD8 double-positive (DP) stage, and these immature thymocytes have double-strand breaks (DSBs) created by RAG enzymes in TCR genes during TCR V(D)J recombination. AAV vectors enter thymocytes through receptor-mediated endocytosis, and the released AAV and transgene DNA in the nucleus is integrated into TCR genes close to the DSBs. The thymocytes with unsuccessful TCR formation owing to the AAV transgene integration will die. The transduced thymocytes with a functional TCR will undergo the selection process, and the positively selected thymocytes will develop into CD4 or CD8 single-positive (SP4 or SP8) T cells in the medulla and eventually be released into the peripheral lymphoid tissue.
IEIs are a heterogeneous group of inherited immune disorders often associated with significant morbidity and mortality. Allogeneic hematopoietic stem cell transplantation is the standard treatment for most severe IEIs. However, allogeneic hematopoietic stem cell transplantation requires a suitable donor and carries the risk of graft failure, graft rejection, graft-versus-host disease, and complications related to pretransplant myelosuppressive and posttransplant immunosuppressive agents. GT can overcome most or all of these disadvantages, and has been used in the treatment of some IEIs with decent clinical outcomes. The current GTs for IEIs are ex vivo GT, genetically modifying patients’ hematopoietic stem cells ex vivo using gammaretroviral vectors or lentiviral vectors.7,8 The procedure of ex vivo GT is complex and requires an advanced infrastructure, which is not widely available. In addition, lymphodepletion preconditioning for ex vivo GT has side effects and complications. It will be of great benefit to correct genetic defects in vivo. Given the findings of Calabria et al, IT AAV GT can be a good approach for treating IEIs primarily due to defects in T lymphocytes, such as CD40 ligand deficiency, immune dysregulation polyendocrinopathy enteropathy X-linked syndrome, and cytotoxic T-lymphocyte antigen 4 insufficiency.
CAR T-cell therapy is the most successful and innovative cellular cancer treatment in modern oncology. It genetically modifies a patient's own T cells ex vivo to generate CAR T cells that express a synthetic receptor that binds to a tumor antigen. CAR T cells are infused back into patients to attack and kill cancer cells. Good therapeutic responses and clinical outcomes have been demonstrated in CAR T-cell treatments for B-cell malignancies.9 However, the high cost and complex procedure to generate CAR T cells ex vivo limit its wide application. The generation of in vivo CAR T cells may simplify the process and make CAR T-cell therapy easier to perform and less expensive. Nawaz et al found that infusing AAV-CAR into a humanized tumor mouse model of human T-cell leukemia could generate enough functional in vivo CAR T cells to cause tumor regression.10 Given the features and advantages of IT AAV gene delivery discussed above, IT delivery of AAV-CAR could be a more effective way to generate in vivo CAR T cells. Because the targeted cells are immature thymocytes, CAR T cells generated by IT AAV-CAR delivery may last longer in the body than the traditionally generated CAR T cells.
It is too early to predict the clinical use of IT AAV gene delivery. However, as an intriguing idea with the support of promising preclinical study results, IT AAV GT deserves further study and may eventually become a useful treatment for certain human diseases.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal