In this issue of Blood, Zeidan et al present updated response criteria for myelodysplastic syndromes (MDS),1 adding to an exciting year of advances for MDS that began with the long-awaited molecular update to the International Prognostic Scoring System2 and not 1, but 2 new classification systems for MDS courtesy of the World Health Organization (WHO)3 and the International Consensus Classification (ICC) committee.4 The article by Zeidan et al presents a consensus proposal revising the International Working Group (IWG) response criteria for higher risk MDS. Other than a 2018 update focused primarily on lower risk MDS,5 the last major revision to the IWG response criteria for MDS were released in 2006.6 The novel IWG 2023 criteria take advantage of what we have learned since then from a multitude of clinical trials and discoveries about the genetic underpinnings of MDS to create more clinically relevant and impactful response definitions for future studies.
The value of agreed-upon consensus response criteria for MDS is difficult to overstate. MDS are highly heterogeneous disorders with varied clinical presentations and disparate outcomes. Standardization of diagnostic subtypes and risk-stratification tools helps define patient populations more uniformly, but how we assess response to treatment is even more critical. Shared response criteria allow for a cleaner comparison of benefit across clinical studies of different agents, in different regions, and at different times and can also serve as surrogate markers for our most important clinical endpoints including overall survival (OS). As earlier endpoints, standardized responses can advance the development of promising agents, even leading to accelerated approvals, while halting programs with little chance of success. Common response criteria also form a language we use to speak to patients, setting expectations for treatments that we can balance against their risk. To these ends, the IWG 2023 response criteria for MDS represent an important advance.
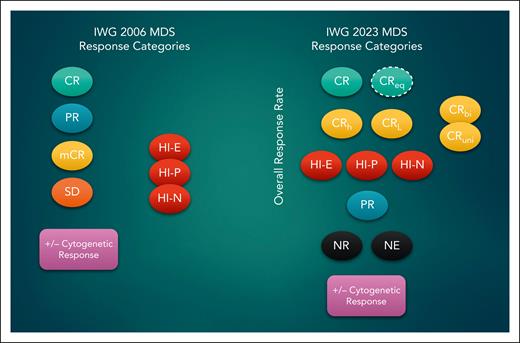
To better understand the revisions in the IWG 2023 criteria, one needs to briefly review the IWG 2006 criteria.6 In this system, MDS patients can achieve a complete remission (CR), defined as bone marrow blasts ≤5% and 0% circulating blasts with a hemoglobin (Hb) >11 g/dL, platelets ≥100 × 109 per liter, and absolute neutrophil counts (ANC) ≥1.0 ×109 per liter. A marrow complete response (mCR) has the same bone marrow blast cutoff (≤5%) but with 1 or more blood count measures falling short of CR criteria. Overall response rates (ORRs) in clinical trials often include the mCR rate, particularly in early phase studies hoping to use the ORR as a surrogate marker of eventual benefit and likelihood of meeting approvable endpoints in later phase trials. Yet mCR without hematologic improvement has been repeatedly shown to not correlate with overall survival and to often predict survival that is indistinguishable from that of nonresponding patients with stable disease.7 Yet, patients achieving an mCR are not uniform. Some may have little or no count recovery, whereas others might be just shy of the CR blood count criteria, and this latter group may still have clinical benefit.
The IWG 2023 criteria have taken several steps to address this concern, including abolishing the mCR response option and creating new response categories (see figure). The Hb level required for CR is also lowered to 10 g/dL, representing a threshold unlikely to result in symptomatic anemia or transfusion dependence while including patients who otherwise might have been relegated to an mCR by IWG 2006. Second, the IWG 2023 formally defines several “less-than-CR” criteria that nevertheless include meaningful improvements in blood counts. The most important of these, in my opinion, is CR with partial hematologic recovery (CRh), which requires platelet counts ≥50 × 109 per liter and ANC ≥0.5 × 109 per liter in addition to meeting CR bone marrow blast criteria. One retrospective study indicates that unlike mCR, MDS patients who achieve a CRh have an OS that is comparable to that of patients achieving an unqualified CR.8 This mirrors experience with CRh in patients with acute myeloid leukemia (AML) and has been used as a surrogate endpoint for several AML-directed therapies approved recently.9 The IWG 2023 criteria for CRh are exactly those adopted by the European Leukemia Network for AML in their revised 2022 criteria.10 This alignment is particularly important given that both the WHO and the ICC stressed the continuum between MDS with excess blasts and AML, with the ICC formally redefining patients with 10% to 19% blasts as having MDS/AML overlap.3,4 The intent by both groups is to expand the eligibility of patients with oligoblastic MDS to receive AML therapy and participate in AML trials, given its equally poor outcomes.
Response categories in the IWG 2006 and 2023 consensus criteria. CRbi, CR with bilineage recovery; CReq, CR equivalent; CRL, CR with limited count recovery; CRuni, CR with unilineage recovery; HI, hematologic improvement; HI-E, HI-erythroid; HI-N, HI neutrophil; HI-P, HI platelet; NR, no response; NE, not evaluable; PR, partial remission; SD, stable disease.
Response categories in the IWG 2006 and 2023 consensus criteria. CRbi, CR with bilineage recovery; CReq, CR equivalent; CRL, CR with limited count recovery; CRuni, CR with unilineage recovery; HI, hematologic improvement; HI-E, HI-erythroid; HI-N, HI neutrophil; HI-P, HI platelet; NR, no response; NE, not evaluable; PR, partial remission; SD, stable disease.
The IWG 2023 criteria also recognize CR with limited count recovery (CRL) as another “less-than-CR” response, identifying patients achieving CR blood count criteria in only 1 or 2 cell lines, described as CRuni and CRbi, respectively. Because MDS patients at higher risk may not have excess blasts, a “CR-equivalent” response can be reported separately for these patients if they achieve a complete cytogenetic response and meet CR criteria for blood counts. In contrast to response criteria for AML and other myeloid malignancies, the concept of a molecular response is not defined because the value of mutational clearance is less clear in MDS. The authors recommend assessing this metric in clinical studies, as it may form the basis of future response criteria when supported by data.
Overall, the revisions made to the MDS response criteria will allow for greater nuance in response assessment while avoiding pitfalls we now know are not associated with longer term clinical benefit. Hopefully, these welcome updates will expand therapeutic options for MDS patients and improve our ability to identify the most promising treatments earlier in their development, all while we learn the lessons we will need for the next revision.
Conflict-of-interest disclosure: R.B. is employed by and has equity in Aptose Biosciences; is a consultant for Bristol Myers Squibb and for Servier; is chair of the data and safety monitoring boards of Gilead and of Epizyme; and receives research funding from Takeda.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal