In this issue of Blood, Hammond et al1 present their results on quantifying the proliferation kinetics and cell cycle progression of single, highly purified human hematopoietic stem cells (HSCs) isolated from cord blood and from young adult and aged healthy donors, to study how age-related changes affect HSC activation, signaling, and proliferation in response to growth-factor stimulation. They show that aged HSCs are less sensitive to growth-factor stimulation and are more difficult to activate than young HSCs.
The extensive capacity of HSCs to self-renew and differentiate is critical to regeneration of the blood system throughout life. But HSC potential is limited and declines progressively with age and proliferative stress.2 To prevent stem cell exhaustion, HSCs are quiescent and rarely divide.3 When needed during insults, such as inflammation, mitogenic signals from the microenvironment awaken and instruct HSCs to divide. A well-established finding is that HSC activation and proliferation rely on growth-factor stimulation, but insights into how age-related changes in human HSCs alter the response to growth factors have been lacking.
HSCs are quiescent, reside in the G0 phase of the cell cycle, contribute little to steady-state hematopoiesis,3 and function as a backup to ensure lifelong hematopoiesis. Waking of HSCs requires the activation of signaling pathways that regulate cell cycle entry and progression, processes that are induced by growth factors, which activate Akt signaling and other pathways. Akt signaling positively regulates cell survival, growth, and the G0/1 and G1/S cell cycle transition by phosphorylation of the cell cycle regulators cyclin-dependent kinase (CDK) 4 and 6 and CDK2.
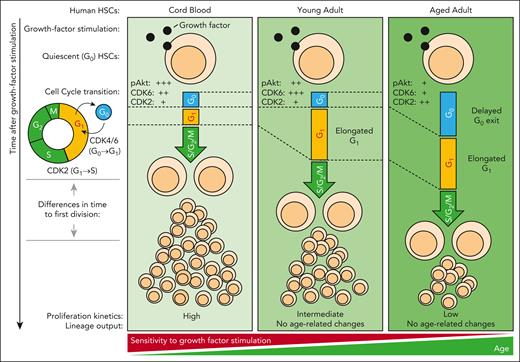
Using single-cell analyses of human HSCs, Hammond et al show that aged HSCs have delayed proliferation kinetics after growth-factor stimulation in vitro. They first isolated highly purified CD49f+ HSCs from cord blood, and young and aged adults, cultured them on mouse stroma cells engineered to support human HSC survival and differentiation, and found an age-related delay in colony growth with minor or no alterations in differentiation. They then sorted and cultured single HSCs in stroma-free conditions, tracked the proliferation kinetics of HSC clones, and quantified the time to first division and the proliferation kinetics of their progeny. These results showed that aged HSCs, irrespective of the culturing systems, have delayed proliferation kinetics compared to those of young adult bone marrow and cord blood-derived HSCs. Interestingly, this delay is inherited by HSC daughters, suggesting that regulation of age-related changes in HSC proliferation is a cell-intrinsic property. To obtain further insight, they next combined suspension cell-capture4 and cyclic immunofluorescence analysis5 to quantify the multiplexed expression of 7 molecular markers, including CDK6 and CDK2. Multiplexed profiling of HSC cell cycle kinetics after growth-factor stimulation revealed that age-related changes in proliferation are correlated with a delayed G0 exit in aged HSCs, and with a prolonged G1 phase in young and aged adult HSCs, respectively, differences that were further exaggerated under conditions of a low concentration of growth factors. Using phosphoflow, they then found an age-related progressive reduction of Akt phosphorylation, and they propose a model in which cell-intrinsic age-related changes desensitize HSCs to growth-factor stimulation (see figure).
Aged HSCs are desensitized to growth-factor stimulation. Age-related changes in young adult and aged HSCs lead to a reduction in Akt signaling, G1 elongation, and reduced proliferation after growth-factor stimulation. These changes are exaggerated in aged HSCs, which show a delayed G0 exit. pAkt indicates the phosphorylation and activation of Akt. +++, ++, and + correspond to high, intermediate, and low levels of pAkt, CDK6, and CDK2 protein, as reported in Hammond et al, in freshly isolated HSCs before growth-factor stimulation. G0, G1, S, G2 and M indicate cell cycle phases. S/G2/M indicates combined duration of S, G2 and M cell cycle phase.
Aged HSCs are desensitized to growth-factor stimulation. Age-related changes in young adult and aged HSCs lead to a reduction in Akt signaling, G1 elongation, and reduced proliferation after growth-factor stimulation. These changes are exaggerated in aged HSCs, which show a delayed G0 exit. pAkt indicates the phosphorylation and activation of Akt. +++, ++, and + correspond to high, intermediate, and low levels of pAkt, CDK6, and CDK2 protein, as reported in Hammond et al, in freshly isolated HSCs before growth-factor stimulation. G0, G1, S, G2 and M indicate cell cycle phases. S/G2/M indicates combined duration of S, G2 and M cell cycle phase.
Although these findings provide novel insight into how aging affects human HSC behavior in response to growth factors, many questions remain about the precise molecular changes causing the reduced sensitivity to mitogenic signals in aged HSCs. Hammond et al report a reduction in Akt signaling in aged HSCs, but what exactly causes the drop in Akt phosphorylation and the desensitization to growth factors remains unclear. The strength and kinetics of Akt activation described by the authors, however, provide important clues for future studies. Although the growth-factor stimulation of young adult and aged HSCs activates Akt briefly, Akt in cord blood HSCs is maintained for 15 to 30 minutes. Akt is therefore quickly dephosphorylated after activation in aged HSCs, suggesting that PTEN (phosphatase and tensin homologue), an important regulator of HSC function and a negative regulator of Akt is either upregulated or more active during HSC aging. However, because Akt activity shortly after growth-factor stimulation is also lower in young adult and aged HSCs, future studies also should investigate whether changes occur upstream of Akt, such as age-related changes in growth-factor receptor expression. If such changes do occur, what regulates the levels of PTEN and/or the growth-factor receptors? One possibility is that these changes are mediated by changes in endosomal trafficking, recycling, and degradation of receptors in lysosomes, which are now recognized as important regulators of signaling, cell fate, quiescence, and aging.6,7
Another avenue that requires further investigation is the precise role of CDK6 during HSC aging. As expected based on previous reports,8 aged HSCs with lower CDK6 protein levels show a delayed G0 exit. However, young adult HSCs, despite expressing more CDK6 than do cord blood–derived HSCs, exit G0 with similar kinetics, suggesting that additional mechanisms are involved in regulating the G0 exit in HSCs. Although no overt differences in lineage output were found by Hammond et al, future studies need to clarify whether growth-factor desensitization is a common feature of all HSCs or rather is restricted to an HSC subpopulation that expands with age and might respond differently, as shown to be the case in mice.9 Also important is to validate that aged HSCs isolated from “healthy” donors are free from mutations associated with clonal hematopoiesis.
In general, more work on aged human HSCs is needed to better understand how aging affects HSC behavior. Future studies need to determine whether the sensitivity to growth-factor stimulation in aged HSCs can be restored to rejuvenate HSCs. Studying signaling activity in mutant HSCs from individuals with clonal hematopoiesis might provide important new insights into the early steps of disease initiation and progression.
Highly purified human HSCs are extremely are. Novel, sensitive, single-cell approaches capable of multiplexing are therefore crucial to learn more about these precious samples. Recent developments in single-cell dynamics quantification10 and tracking will be instrumental moving forward, because imaging requires very few cells to make statistically sound conclusions, captures cellular heterogeneity, and can deconvolute unsynchronized cell behavior.7 Improving our understanding of signaling dynamics, heterogeneity, and crosstalk in young and aged HSCs will guide the development of novel culturing strategies to expand and rejuvenate HSCs more efficiently.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal