In this issue of Blood, Hardouin et al report on the development of an adenine base-editing approach capable of correcting the most common β-thalassemia mutation in the eastern Mediterranean, IVS1-110.1
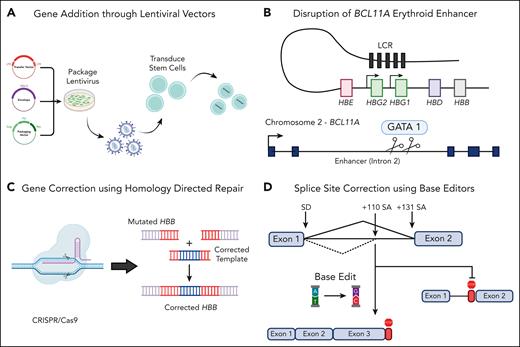
β-Thalassemia, a common recessive genetic disorder characterized by reduction in β-globin production, results in mild to severe transfusion-dependent anemia. There have been several recent advances in the field of gene therapy to combat β-thalassemia through lentiviral vector and CRISPR/Cas9-based gene therapies (see figure); however, these therapies have some inherent safety concerns and a high cost of goods, a challenge Hardouin et al tackled.
Schematic of various gene therapy strategies to treat β-thalassemia. (A) The use of lentiviral vectors to incorporate a full-length HBB gene or shmiRs targeting BCL11A for the upregulation of HbF. The use of CRISPR/Cas9 machinery can either knock out BCL11A to upregulate HbF (B) or incorporate a corrected HBB cassette with HDR machinery (C). Finally, adenine base editors can be used to convert A to G nucleotides to correct single-point mutations or incorrect splice sites, such as IVS1-110 (D). Created with BioRender.com.
Schematic of various gene therapy strategies to treat β-thalassemia. (A) The use of lentiviral vectors to incorporate a full-length HBB gene or shmiRs targeting BCL11A for the upregulation of HbF. The use of CRISPR/Cas9 machinery can either knock out BCL11A to upregulate HbF (B) or incorporate a corrected HBB cassette with HDR machinery (C). Finally, adenine base editors can be used to convert A to G nucleotides to correct single-point mutations or incorrect splice sites, such as IVS1-110 (D). Created with BioRender.com.
In August 2021, the Food and Drug Administration (FDA) approved betibeglogene autotemcel, an ex-vivo lentiviral vector gene therapy for patients with transfusion-dependent β-thalassemia. This treatment was based on data from a phase 3 trial in which 32 of 36 patients attained transfusion independence after receiving the gene therapy. Although largely successful at ameliorating the disease phenotype, this lentiviral gene therapy (and many before it) suffers from a high cost of goods, owing to its long vector size and relatively low-titer and suboptimal gene transfer. Other efforts at targeting β-thalassemia have been attempted on the gene-editing front using CRISPR/Cas nuclease-induced double-stranded break (DSB) to repair disease-causing mutations in the gene for β-globin (HBB) by means of homology-directed repair.2,3 More recently, Cromer et al have attempted to correct the imbalance of α- and β-globin present in patients with β-thalassemia by integrating a β-globin transgene downstream of the HBA2 promoter of α-globin to not only knock down excess α-globin production but also increase the dearth of β-globin expression.4 Although these methods are effective at producing properly regulated β-globin, they are hindered by low levels of homology-directed repair (HDR), DSB-induced toxicity via p53-mediated damage response, chromosomal translocations, large deletions, and chromothripsis.5
As an alternative approach to tackling β-thalassemia, researchers have attempted to elicit the condition of hereditary persistence of fetal hemoglobin (HPFH) within patients. Patients with HPFH have elevated levels of fetal hemoglobin (HbF), resulting in milder clinical manifestations of anemia. To recapitulate HPFH, researchers either deleted 13kb of the β-globin locus or, more recently, used a nonhomologous end joining-based CRISPR/Cas9 knockout of the BCL11A erythroid enhancer required for repression of HbF in mature erythroid cells.6 Liu et al at Boston Children’s Hospital have generated a lentiviral vector containing a microRNA-adapted short hairpin RNA that targets BCL11A transcripts to increase levels of γ-globin and HbF.7 However, as with the previously described lentiviral vector and DSB-mediated CRISPR/Cas9 technologies, these strategies have safety concerns and/or a high cost of goods.
Hardouin et al used adenine base editors (ABEs) for targeted correction of the IVS1-110 β-thalassemia–causing mutation. ABEs are unique in their editing approach compared with previous CRISPR/Cas nucleases because they allow for accurate DNA repair without inducing DSBs. This correction results from the ABE machinery, which contains a Cas9 nickase and an adenine deaminase (capable of converting A to G as seen in the IVS1-110 mutation). The authors assess 6 different guide RNAs (gRNAs) alongside each of the ABE8e, NG-ABE8e, and SpRY-ABE8e editors before determining a candidate gRNA1/SpRY-ABE8e combination capable of correcting 90% of the IVS1-110 HBB mutant alleles. Although base editors can generate bystander mutations (identical edits nearby the target site), the 2 observed bystanders described by Hardouin et al were intronic and had no effect on HBB expression and erythroid differentiation. Furthermore, transfected patient hematopoietic stem and progenitor cells (HSPCs) demonstrated restored β-globin splicing and increased levels of HbA and β-globin chains and elicited appropriate regulation and rescue of erythroid precursors. NBSGW mice transplanted with transfected β-thalassemic HSPCs contained >70% phenotype correction and showed restoration of α/non-α ratio and increased levels of β-globin chains by high-performance liquid chromatography.
The use of highly precise ABEs to correct the single nucleotide mutation responsible for the IVS1-110 mutation is an incredible advance for the field of gene therapy. Hardouin et al not only have demonstrated near wild-type restoration of HbA expression in vitro and in vivo but also done so without causing any DSBs. The high-level targeted gene correction seen by ABEs not only supersedes the lower efficiency exhibited by HDR-based gene editing and lack of regulated expression exhibited by lentiviral vectors but also circumvents the toxic events expected by Cas9 nuclease-mediated DSBs. Although ABEs are limited by their on-target bystander edits (base-pair changes to other nucleotides within the region bound by the ABE protein), the targeting window is limited, and their frequency can be reduced (with some reduced on-target conversion) with more precise ABEs such as ABEmax.8
Unfortunately, as demonstrated in this work, base editors are limited to single nucleotide changes (and currently, transitions but not transversions), preventing the generation of suitable therapies for a variety of disorders targetable by HDR-based gene-editing strategies or lentiviral vectors. Although prime editing, a newer technology capable of inserting or deleting larger fragments of DNA without the need for DSBs, shows promise, it currently lacks the efficiency necessary for therapeutic benefit of hematopoietic disorders.9 Furthermore, the major difficulty of commercial adaptation for this therapy, and many like it, stems from its rare frequency. The IVS1-110 mutation composes ∼30% of eastern Mediterranean patients, only a small portion of the world’s β-thalassemia patients.1 To reach the clinic and overcome the hurdles of manufacturing, funding, and regulation that rare and ultra-rare diseases face, policies must be put in place streamlining their development processes. The National Institutes of Health and FDA’s Bespoke Gene Therapy Consortium is one such evolution necessary to overcome this hurdle, paving the way for a potential platform regulatory review (ie, same base editor, different gRNA) that relies on previous knowledge to streamline development and regulation of rare therapies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal