Gain of chromosome 21 (+21) is one of the most frequent alterations seen in leukemia; yet its impact on normal and malignant hematopoiesis is still incompletely understood. In this issue of Blood, Gialesaki et al1 provide compelling evidence for the oncogenic role of a disequilibrium of the transcripts expressed from the chromosome 21 gene RUNX1, a previously unknown “layer” of imbalance in myeloid leukemia associated with children with Down syndrome (ML-DS).
ML-DS development is a well-known model of multistep leukemogenesis, starting with the perturbation of hematopoiesis during fetal life, which continues after birth in all neonates with Down syndrome (constitutive trisomy 21); the development of a preleukemic syndrome named transient abnormal myelopoiesis (TAM) in 10% to 20% of neonates with Down syndrome, which regresses during the first weeks of life; and the progression from TAM to ML-DS seen in approximately 20%.2 Although TAM is associated with somatic GATA1 mutations that encode a short isoform named GATA1s,3 and progression towards ML-DS is associated with other alterations affecting the function of cohesin complex components (such as STAG2 and RAD21), epigenetic regulators (such as EZH2 and SUZ12), or signaling effectors (such as JAK2/3 and MPL),4 the impact of the trisomy 21 in TAM/ML-DS leukemogenesis, alone or in concert with these somatic mutations, remains unclear. Over the past 2 decades, many experimental models (transgenic mice, cell lines, fetal liver cells, and induced pluripotent cells) were used to dissect this stepwise TAM/ML-DS development.4-8 From these studies, a short list of dosage-sensitive chromosome 21 genes and regulatory elements shown to promote leukemogenesis based on the ‘3 copies vs 2’ hypothesis (ERG, DYRK1A, and miR-125b-2) was generated (see figure).5,9,10
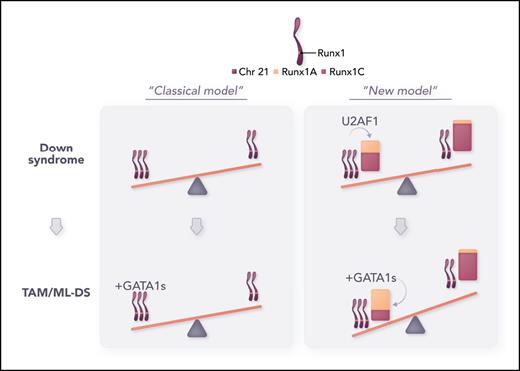
Refining the model of dosage imbalance in TAM/ML-DS. From this study, the impact of trisomy 21 on TAM/ML-DS pathogenesis has been refined from the “classical” model (gene dosage effect, left panel) to a “new” model in which the combination of 3 chromosome 21 and a disequilibrium of the RUNX1A/RUNX1C transcripts promotes myeloid leukemia in children with DS (right panel). Professional illustration by Somersault18:24.
Refining the model of dosage imbalance in TAM/ML-DS. From this study, the impact of trisomy 21 on TAM/ML-DS pathogenesis has been refined from the “classical” model (gene dosage effect, left panel) to a “new” model in which the combination of 3 chromosome 21 and a disequilibrium of the RUNX1A/RUNX1C transcripts promotes myeloid leukemia in children with DS (right panel). Professional illustration by Somersault18:24.
Here, Gialesaki et al elegantly establish that the chromosome 21 gene RUNX1, which is frequently altered in hematologic malignancies, is implicated in TAM/ML-DS but not as expected through the bona fide increased genomic material due to trisomy 21. Briefly, following a CRISPR-based drop-out screen to identify new chromosome 21 genes, the authors found a disequilibrium of the RUNX1 transcripts in T21/TAM/ML-DS samples, characterized by the increased ratio of the RUNX1A isoform (truncated from its transactivation domain) over RUNX1C (see figure “New model”). In subsequent experiments, they confirmed that RUNX1A ectopic expression promotes proliferation and growth of immature megakaryocytic progenitors compared with RUNX1C and functionally cooperates with GATA1s to drive a TAM/ML-DS–like phenotype both in vitro and in vivo.
Mechanistically, mass spectrometry and CUT&RUN experiments demonstrate that the RUNX1A isoform, but not RUNX1C, interacts with the MYC cofactor MAX and binds DNA to upregulate the expression of MYC- and E2F-targets, as well as known oncogenic drivers such as Lyl1 and Evi1. In contrast, RUNX1C interacts with GATA1wt and GATA1s to induce megakaryocytic genes, a differentiation signature that is downregulated when RUNX1A is overexpressed. Notably, because RUNX1A interacts with RUNX1C, these results support a model in which increased levels of the RUNX1A isoform not only actively upregulates MYC/E2F-induced proliferative program but also perturbs RUNX1C/GATA1-induced megakaryopoiesis. In line with this model, genetic or pharmacologic inhibition of MYC/MAX complexes, downstream of RUNX1A, decreases cellular growth and induces differentiation of ML-DS blasts.
Although the exact mechanism(s) governing the altered level of expression of RUNX1 transcripts in TAM/ML-DS is still unknown, this study suggests that it may be at least in part intrinsic to trisomy 21 through the increased expression of the chromosome 21 gene U2AF1, which encodes a splicing factor subunit often mutated in myeloid malignancies (myelodysplastic syndrome and acute myeloid leukemia [AML]). Preliminary data show that GATA1s also regulate this imbalance, suggesting that a feed forward loop may occur during the stepwise pathogenesis of TAM/ML-DS itself. Strikingly, several subtypes of AML, with or without gain of the chromosome 21, have a high RUNX1A/RUNX1C ratio and are also sensitive to MAX interference and pharmacologic inhibition of MYC/MAX complexes. This emphasizes the broad therapeutic potential of the study and warrants further investigation of mechanisms that can promote this disequilibrium and how they cooperate to drive leukemogenesis in non–Down syndrome cases. It would also be of great interest to assess whether non-myeloid leukemia harboring complete or partial gain of the chromosome 21 exhibits an altered RUNX1A/C ratio and whether this is pathogenic in different hematopoietic lineages.
Altogether, Gialesaki et al show for the first time that ML-DS blasts are dependent on RUNX1 and demonstrate that increased level of RUNX1A has a pivotal role in Down syndrome leukemogenesis. Notably, this feature is not unique to ML-DS pathogenesis, highlighting a potentially underestimated role for these onco-transcripts. Therefore, this work emphasizes the need to seek new alternative transcripts in hematologic malignancies and to identify their downstream effectors and functional consequences on tumor development.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal