Abstract
Introduction: Patients with lower-risk myelodysplastic syndromes (LR-MDS) receiving luspatercept in the phase 3 MEDALIST trial previously reported durable episodes of red blood cell (RBC) transfusion independence (RBC-TI) lasting ≥ 8 weeks, with a median cumulative duration of approximately 20 months (Fenaux P, et al. J Clin Oncol 2022;40[16_suppl]:7056). The baseline characteristics of patients who receive long-term luspatercept treatment, as well as the benefits of long-term treatment, warrant further investigation. Here, we report the effect of longer duration of treatment on response, and the baseline characteristics of patients with longer durations of treatment, in patients with LR-MDS receiving luspatercept in the MEDALIST long-term follow-up study.
Methods: Eligible patients were ≥ 18 years of age, had LR-MDS requiring RBC transfusions, and were ineligible for, intolerant to, or refractory to erythropoiesis-stimulating agents (ESAs). Patients receiving luspatercept were stratified by duration of treatment, as follows: 0-24, > 24 to 48, > 48 to 144, and > 144 weeks. Efficacy outcomes included: RBC-TI ≥ 8 or 16 weeks; modified hematologic improvement-erythroid (mHI-E) response, based on the International Working Group 2006 definition, over any consecutive 56-day period; and time to acute myeloid leukemia (AML)/high-risk (HR) MDS progression from diagnosis.
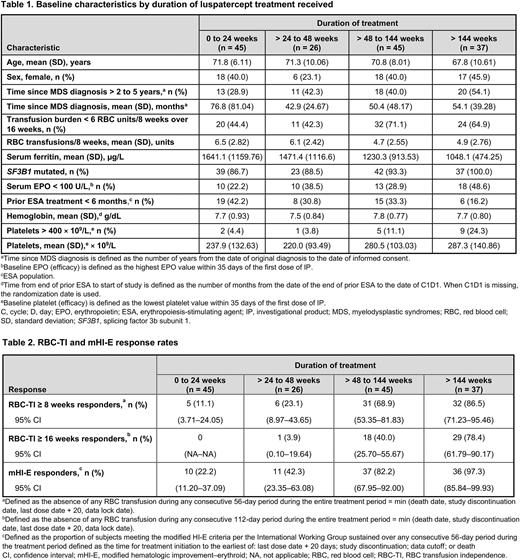
Results: As of January 15, 2021, of 153 patients randomized to luspatercept, 45 (29.4%), 26 (17.0%), 45 (29.4%), and 37 (24.2%) patients remained on treatment during weeks 0-24, > 24 to 48, > 48 to 144, and > 144, respectively. Baseline characteristics stratified by duration of treatment are shown in the Table. Patients treated for > 48 weeks were younger; had lower baseline transfusion burden, serum ferritin (SF), and erythropoietin (EPO) levels; were more likely to have mutated SF3B1; and less likely to have received ESAs in the 6 months prior to study. Patients treated for ≤ 24 weeks had a longer time since MDS diagnosis (Table 1).
The majority of patients received the maximum luspatercept dose level regardless of duration of treatment, with 53.3%, 69.2%, 80.0%, and 73.0% of patients receiving 1.75 mg/kg luspatercept during weeks 0-24, > 24 to 48, > 48 to 144, and > 144, respectively. Median (range) time to first dose increase was generally higher with longer duration of treatment (43 [40-106] vs 91 [41-1023] days at 0-24 and > 144 weeks, respectively).
Higher rates of RBC-TI ≥ 8 weeks, RBC-TI ≥ 16 weeks, and mHI-E ≥ 8 weeks were associated with longer durations of treatment (Table 2). Median (range) cumulative duration of RBC-TI ≥ 8 weeks was greater with longer duration of treatment: 66.0 (57-166), 88.5 (62-229), 373.0 (59-936), and 1227.5 (59, 1767) days for patients with a duration of treatment of 0-24,
> 24 to 48, > 48 to 144, and > 144 weeks, respectively.
Progression to HR-MDS/AML occurred in 3 (6.7%), 2 (7.7%), 7 (15.6%), and 1 (2.7%) patient with a duration of treatment of 0-24, > 24 to 48, > 48 to 144, and > 144 weeks, respectively. The median (range) time from original MDS diagnosis to HR-MDS/AML progression was 66.2 (57.2-223.6), 41.9 (18.8-64.9), 49.0 (33.1-97.6), and 56.7 (56.7-56.7) months for patients with a duration of treatment of 0-24, > 24 to 48, > 48 to 144, and > 144 weeks, respectively.
Conclusions: Patients continuing treatment for > 48 weeks were younger and had lower baseline transfusion burden, SF, and EPO levels. Higher rates of RBC-TI and mHI-E response led to longer durations of treatment, and patients with longer durations of treatment were more likely to receive the maximum luspatercept dose level. There were no safety signals in terms of progression to AML/HR-MDS occurring according to the expected prognostic risk profile for these patients. These findings illustrate that patients with LR-MDS receiving long-term luspatercept treatment continue to experience durable responses.
Disclosures
Platzbecker:Silence Therapeutics: Honoraria; Geron: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Janssen: Honoraria; Jazz: Honoraria; BMS/Celgene: Honoraria; Abbvie: Honoraria. Santini:Otsuka: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Menarini: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria; Novartis: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Srvier: Membership on an entity's Board of Directors or advisory committees. Komrokji:PharmaEssentia, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Acceleron Pharma: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau; CTI BioPharma, Innovent: Honoraria, Membership on an entity's Board of Directors or advisory committees; Taiho Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Zeidan:Celgene/BMS, AbbVie, Pfizer, Boeringer-Ingelheim, Trovagene, Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, Amgen, Otsuka: Consultancy, Honoraria, Research Funding; Astex, Medimmune, Astrazeneca, ADC Therapeutics: Research Funding; Novartis, Cardiff Oncology, Pfizer: Other: Travel Support; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie: Consultancy, Honoraria, Other: Advisory Board; Celgene/BMS, Novartis, AbbVie, Gilead, Kura, Loxo Oncology, Geron: Other: Clinical Trial Committee; Gilead, Kura, Loxo Oncology: Consultancy, Honoraria, Other: Clinical Trial Committee; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie, Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Astex, Pfizer, Medimmune/AstraZeneca, ADC Therapeutics: Research Funding; Jazz, Agios, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, Beyondspring, Gilead, Kura, Tyme, Janssen, Syndax, Geron, Ionis, Epizyme: Consultancy, Honoraria; Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Gilead, Kura, Loxo Oncology, Otsuka, Jazz, Agios, Acceleron, Astellas, Daiichi-Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Ionis, Epizyme, Janssen, Syndax, Genentec: Consultancy, Honoraria, Other: Advisory Boards. Garcia-Manero:Astex: Consultancy, Honoraria, Research Funding; Aprea: Honoraria; Genentech: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Gilead Sciences: Research Funding; Curis: Honoraria, Research Funding; Acceleron Pharma: Consultancy. Buckstein:Takeda: Research Funding; BMS: Honoraria, Research Funding; Taiho: Honoraria, Research Funding. Oliva:BMS: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy; Apellis: Consultancy, Honoraria; Sobi: Honoraria; Amgen: Honoraria, Speakers Bureau; Novartis: Patents & Royalties: HM PRO, Speakers Bureau; Daiichi: Consultancy. Miteva:BMS: Current Employment. Pozharskaya:BMS: Current Employment. Ha:BMS: Current Employment. Nadal:BMS: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Fenaux:BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Celgene/BMS: Honoraria, Research Funding; Syros Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal