Abstract
Background: Myelofibrosis is a rare bone marrow disorder that is characterized by clonal proliferation of myeloid cells and bone marrow fibrosis. Myelofibrosis belongs to the group of BCR/ABL1 negative myeloproliferative neoplasms. Patients with myelofibrosis and severe thrombocytopenia (platelet counts <50x109/L) are generally older, with more advanced disease and increased risk of bleeding, higher rates of anemia and complex/unfavorable cytogenetics, and shortened overall survival compared to patients with higher platelet counts. So far, treatment options for patients with myelofibrosis and severe thrombocytopenia are limited due to the high incidence of treatment-related thrombocytopenia when other JAK inhibitors are used. Pacritinib is an oral JAK2/IRAK1/ACVR1 inhibitor with demonstrated clinical activity in myelofibrosis in two phase 3 studies and a phase 2 dose-finding study; all included patients with severe thrombocytopenia. The PACIFICA trial (NCT03165734) is designed to confirm efficacy and safety of pacritinib 200 mg twice daily vs physician's choice (P/C) therapy in patients with myelofibrosis and severe thrombocytopenia.
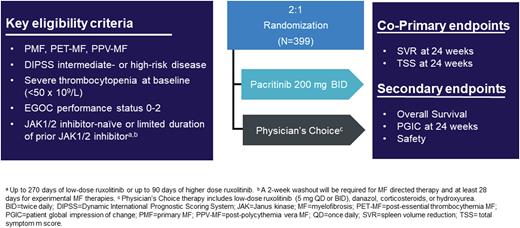
Study Design: PACIFICA is a multinational, multicenter, randomized, controlled phase 3 trial of pacritinib vs P/C in adults with primary or secondary myelofibrosis with DIPSS intermediate- or high-risk disease, ECOG performance status 0-2, platelet counts <50 x 109/L, limited prior JAK2 inhibitor therapy (up to 270 days of low-dose ruxolitinib or up to 90 days of any JAK2 inhibitor), or JAK2 inhibitor naïve, and not candidates for stem cell transplant (Figure 1). Additional exclusion criteria include recent grade ≥2 cardiac or hemorrhagic events, left ventricular ejection fraction <50%, QTc >450 msec, or use of medications that increase risk of hemorrhage or QTc prolongation.
Patients are randomized 2:1 to continuous pacritinib 200 mg twice daily or P/C (low-dose ruxolitinib [no more than 10 mg/day], danazol, corticosteroids, or hydroxyurea). Following regulatory feedback an amendment was initiated, and the study was updated to have co-primary endpoints involving both spleen volume reduction (SVR) and total symptom score (TSS). The co-primary endpoints are the proportion of patients achieving a ≥35% SVR and the proportion achieving a ≥50% reduction in modified TSS from baseline at week 24. To account for co-primary endpoints, the sample size was adjusted from 348 to 399 patients to provide 85% power to detect a difference between pacritinib and P/C. Secondary objectives include Patient Global Impression of Change response at week 24, overall survival, and safety. Tertiary endpoints include leukemia-free survival, hematologic improvement, fatigue improvement (based on PROMIS v.1.0 - Fatigue - Short Form 7a), changes in biomarkers and gene expression, and proportion of patients who experience a major adverse cardiac event (MACE). Given the US approval of pacritinib, PACIFICA is currently enrolling outside of the US at ~100 sites worldwide.
Disclosures
Mascarenhas:Forbius: Research Funding; Merck: Research Funding; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Research Funding; Prelude Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janseen: Research Funding; Merus: Research Funding; AbbVie: Consultancy, Research Funding; Sierra Oncology: Consultancy; GSK: Consultancy; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy; Kartos: Consultancy, Research Funding; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Galecto: Consultancy; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imago: Consultancy; Roche: Consultancy, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Gerds:Imago BioSciences: Research Funding; Bristol Myers Squibb/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Accurate Pharmaceuticals: Research Funding; Kratos Pharmaceuticals: Research Funding; Incyte Corporation: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys/Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kiladjian:AbbVie: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; AOP Orphan: Membership on an entity's Board of Directors or advisory committees. Döhner:BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Research Funding; Astellas: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kronos: Research Funding. Buckley:CTI BioPharma: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Smith:CTI BioPharma: Current Employment, Current holder of stock options in a privately-held company. Craig:CTI BioPharma: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Bedi Singh:CTI BioPharma: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Verstovsek:Celgene: Consultancy, Research Funding; Sierra Oncology: Consultancy, Research Funding; Roche: Research Funding; Protagonist Therapeutics: Research Funding; Incyte: Consultancy, Research Funding; CTI BioPharma Corp.: Research Funding; Genentech: Research Funding; Gilead: Research Funding; PharmaEssentia: Research Funding; NS Pharma: Research Funding; Promedior: Research Funding; ItalPharma: Research Funding; Novartis: Consultancy, Research Funding; Constellation Pharmaceuticals: Consultancy; Blueprints Medicines Corp.: Research Funding; AstraZeneca: Research Funding; Pragmatist: Consultancy. Harrison:Promedior: Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; AOP Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; EHA: Other: Leadership role; MPN voice: Other: Leadership role; Galecto: Consultancy, Membership on an entity's Board of Directors or advisory committees; Keros: Consultancy; Galacteo: Membership on an entity's Board of Directors or advisory committees; Sierra: Honoraria; Incyte: Speakers Bureau; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; Shire: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal