Abstract
Background: The definition of advanced stage classic Hodgkin lymphoma (cHL) may vary, with bulky stage 2A (> 10cm or > 33% of transthoracic diameter otherwise described as 'large mediastinal adenopathy’ (LMA)) and/or stage 2B with risk factors, included in some studies and clinical guidelines. The ECHELON 1 study recently demonstrated improved overall survival (OS) using AVD-brentuximab vedotin (BV) compared with ABVD in stage 3/4 cHL (Ansell et al. NEJM 2022). Similarly, the pediatric combination BV-AVE-PC demonstrated improved event free survival (EFS) over ABVE-PC in a phase 3 trial of 'high risk patients (pts)’ age 2-21 years (y) (AHOD1331), a definition that included stage 2B bulky (LMA) pts, with a significant benefit shown in this subgroup (HR 0.09 (0.01-0.69)) (Castellino et al. ASCO 2022). Thus, re-evaluation of 'advanced stage’ definitions is warranted, especially in the adult population. Herein, we evaluated the long-term outcomes of pts with stages 1/2 bulky and 2B, a group that has been uniformly included in our advanced stage management approach.
Methods: The BC Cancer Lymphoid Cancer Database was screened to identify all pts 16-70 y with 1/2A bulky (≥ 10 cm) and 2B (± bulky) disease treated with ABVD chemotherapy. Since 2005, advanced stage cHL pts in BC have been managed with 6 cycles of ABVD and PET-guided consolidative RT, whereby those with an EOT PET positive (pos) scan received RT. Following report of the RATHL trial in 2015, those pts with an interim PET2 negative (neg) scan had bleomycin omitted from the latter 4 cycles. For the current study, PET scans assessed by the IHP criteria were re-reviewed to assign a Deauville (D) score (PET-neg=D1-3, DX; PET-pos=D4-5). Freedom from treatment failure (FFTF) was measured from diagnosis to relapse/ progression of HL, or death due to HL or treatment toxicity.
Results: From 2005-2020, 295 pts (148 M, 147 F) were identified. By stage: 1A bulky n=2, 2A bulky n=71 (henceforth called 'bulky only’ n=73); 2B n=133; 1B bulky n=3, 2B bulky n=86 (henceforth called stage 1/2B bulky n=89). Median age was 30 y (range 17- 69 y), median mass size 10 cm overall and 11 cm in bulky cases (range 10-20 cm). Of those with bulky disease (n=162), 147 (91%) had a bulky mediastinal mass.
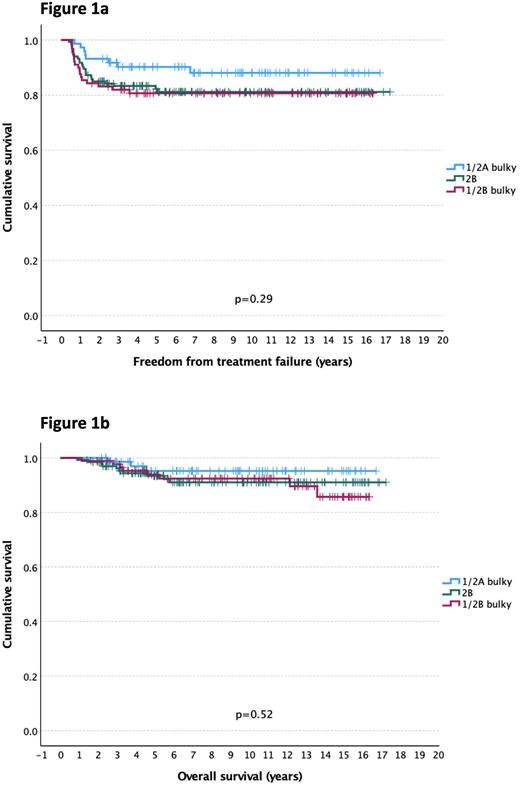
Using the reverse Kaplan-Meier method, median follow-up was 9.3 y (0.9 -17.3 y). The 5 y FFTF and OS for all pts were 84% and 94%. By stage groups, 5 y FFTF estimates were: 90% for bulky only, 82% in 2B, and 81% in 1/2B bulky pts (p=0.29) (Figure 1a), while corresponding 5 y OS estimates were 95%, 93%, and 94%, respectively (p=0.52) (Figure 1b).
An EOT PET was performed in 250 pts and an additional 11 patients were included who only had a PET2-neg scan which was not repeated (total PET scan n= 261). The main reason for not having a PET scan was absence of residual mass on CT scan (21/34); 2 patients had progressive disease (PD) while on treatment (both stage 2B). In total 208/261(80%) had a PET-neg scan (2 pts received RT), and 53/261 (20%) had an EOT PET-pos scan of which, 32 (63%) received RT (mediastinal RT, 30/32). Stage 1/2B bulky cases were more likely to have a PET-pos scan (1/2B 32% vs 2B 18% vs bulky only 10%, p=0.002), and as a result, were more likely to receive RT (1/2B bulky 23% vs 2B 9% vs bulky only 6%, p=0.003). Overall, the 5 y FFTF was superior in PET-neg cases (93% vs 49%, p<0.001 (and 62.5% in PET-pos pts who received RT)), and 5 y OS was 96% vs 88% (p=0.17). For the 259 cases in which the D score was available, 5 y FFTF was similar across PET-neg D categories: DX 95%; D1 92%; D2 94%; D3 93%. For all DX-3, 5y FFTF was 94%, and superior to both D4 70% and D5 17% (p<0.001). Corresponding 5 y OS estimates for DX-D3, D4, and D5, were 96%, 95%, and 77%, respectively, with only the latter demonstrating an inferior prognosis (p=0.005). An EOT D5 PET scan was more common in pts with B symptoms: stage 1/2B bulky cases 12/83, 14.5%; 2B 10/106, 9%; bulky only 2/70, 3% (p=0.048).
Conclusion: Applying an advanced stage management approach yields excellent outcomes, particularly in the 80% of patients with a PET-neg scan. Further, use of a PET-scan limited RT use to only 6% of pts with stage 1/2A bulky disease. However, those with stage 1/2B bulky disease have a higher likelihood of an EOT PET-pos scan and subsequent RT. Further, an EOT PET D5 score was more common in those with B symptoms overall, especially when coupled with bulky disease. This data provides further rationale for consideration of BV with upfront therapy in other advanced stage subgroups.
Disclosures
Villa:AstraZeneca, Roche: Research Funding; Roche, AstraZeneca, Abbvie, Janssen, Kite/Gilead, BMS/Celgene, BeiGene, Kyowa Kirin: Consultancy, Honoraria. Gerrie:AstraZeneca: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Sandoz: Honoraria; Janssen: Honoraria, Research Funding. Venner:Pfizer: Honoraria; BMS: Honoraria; Sanofi: Honoraria; GSK: Honoraria; Takeda: Honoraria; Janssen: Honoraria; FORUS Therapeutics: Honoraria. Slack:Seagen: Honoraria. Scott:Incyte: Consultancy; Janssen: Consultancy, Research Funding; AstraZeneca: Consultancy, Honoraria; Roche: Research Funding; NanoString: Patents & Royalties; Abbvie: Consultancy. Sehn:AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, BMS/Celgene, Debiopharm, Genmab, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Novartis, Sandoz, Seattle Genetics, Servier, Takeda, TG Therapeutics, Verastem: Consultancy; Chugai: Consultancy, Honoraria; Teva, Roche/Genentech: Consultancy, Honoraria, Research Funding; AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, BMS/Celgene, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Sandoz, Seattle Genetics, Servier, Takeda, TG Therapeutics, Verastem: Honoraria. Savage:Beigene and Regeneron: Membership on an entity's Board of Directors or advisory committees; BMS, Janssen, Kyowa, Merck, Novartis, and Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal