Abstract

Therapy related Myeloid Neoplasms (tMNs) constitute 10-20% of newly diagnosed AML, MDS and MDS/myeloproliferative neoplasms (MPN) and have a poor prognosis. t-MNs develop in only a small percentage of patients who receive chemoradiotherapy (CRT). Pathogenic clones surviving genotoxic stress give rise to tMNs. Do factors beyond CRT aid in the clonal evolution? Several medical comorbidities including cardiovascular disease, infections, autoimmunity, organ transplantation etc., are associated with clonal hematopoiesis (CH). We sought to understand if CH-associated comorbidities play a role in CH's evolution to tMN beyond CRT.
A population-based retrospective cohort study was conducted using cancer registries from the SEER Program and Medicare claims database. Descriptive analyses included female adults aged 66-84 who were diagnosed with first primary breast cancer during 2000 to 2014 (followed up through 2015), and survived at least 1 year, as reported to SEER. Using the claims database, individuals were classified as having a medical comorbidity of interest if they had at least one hospital claim, or two physician/outpatient claims at least 30 days apart for that comorbidity. Chi-square tests were used to examine associations between potential risk factors and tMDS/AML. Factors associated with tMDS/AML were then included in the multivariable model. The logistic regression model was assessed for fit, and significance was set at p<0.05.
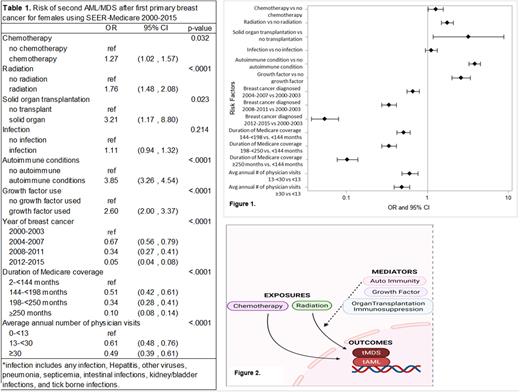
Among the 233,206 female breast cancer (BC) patients, 711 tMDS/AML cases, median age 73 years were identified. As expected, tMDS/AML occurred more often than expected after initial chemotherapy (Odds Ratio; OR=1.27 95% CI=1.02-1.57) and radiation (OR=1.76 95% CI=1.48-2.08) (Table 1 and Figure 1). Patients who had prior solid organ transplantation (SOT) were 3 times more likely to develop tMDS/AML(95% CI=1.17-8.80). Similarly, prior history of autoimmune conditions significantly increased the risk of subsequent tMDS/AML (OR=3.85, 95% CI=3.26-4.54). Use of growth factor significantly increased the risk of subsequent tMDS/AML (OR=2.60; 95% CI=2.00-3.37). Decreased tMDS/AML trends by year of breast cancer diagnosis were noted, which is likely a function of increased early-stage breast cancer diagnoses following widespread mammography initiatives and other sensitive imaging-technique adoption facilitating earlier stage diagnoses. Therefore, less receipt of chemotherapy over time. No other comorbidities (high blood pressure, dyslipidemia, diabetes etc.) were associated with increased risk of subsequent tMDS/AML.
This large-scale US population-based data demonstrates well-established excess tMDS/AML risk following CRT. Additional findings in our study lead to a hypothesis that other mediators/selection pressures are involved in clonal evolution of CH after CRT exposure to develop tMDS/AML (Figure 2). These factors include immunosuppressed state (as therapy for autoimmunity or as prophylaxis to prevent SOT rejection) and G-CSF exposure. We observed similar significant findings in cancer types other than breast cancer; those findings will be presented. The findings warrant reconsideration and modifications to common clinical practices such as the use of G-CSF in solid tumor malignancies as primary prophylaxis to limit neutropenia. We propose that these mediators/selection pressures enhance CH expansion and increase risk of tMDS/AML. Future studies should evaluate this at molecular level longitudinally in patients at risk i.e., patients with CH. Further delineation of these selective pressures and incorporation of CH-inspired clinical risk prediction models to guide CRT, immunosuppression, or G-CSF administration may aid tMDS/AML risk mitigation.
Disclosures
Thota:BMS: Membership on an entity's Board of Directors or advisory committees; Blueprint Medicine: Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
*Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal