Abstract
Background Burkitt lymphoma (BL) is an aggressive B-cell lymphoma that accounts for 50-75% of childhood cancers in sub-Saharan Africa. Recurrent infection with Plasmodium (P.) falciparum is hypothesized to be causally linked to BL, but the epidemiological evidence is limited because it is based on exposures measured after BL onset. The prolonged period before BL onset makes traditional cohort studies impractical. To circumvent this problem, it was proposed that genetic variants that confer genetic resistance to P falciparum, such as heterozygotes for the HBB sickle allele (rs334), could be used to demonstrate a causal link with malaria. However, results from six studies have been conflicting, perhaps, reflecting effects of small sample size, design flaws in the studies or confounding. For example, co-inheritance with other genetic variants, such as a-thalassemia, have been reported to confound the epidemiological associations of severe malaria with rs334. Additionally, resistance against P. falciparum due to "premunition", defined as anti-clinical and anti-parasite resistance due to acquired immunity, could confound genetic associations with BL. To address the hypothesis of whether resistance to malaria protects against BL, we investigated the associations between BL and rs334 and 31 other single nucleotide polymorphisms (SNPs) plus α-thalassemia (hereafter, index malaria resistance SNPs/variants), and premunition, defined as P. falciparum positivity at enrollment, in cases enrolled in Uganda, Tanzania, Kenya, and Malawi, all designated as high malaria burden countries by the World Health Organization.
Methods The study cohort consisted of 800 children (<16 years) with BL and 3845 children without BL enrolled in the Epidemiology of Burkitt Lymphoma in East African Children and Minors (EMBLEM) study (2010-2016) and the Infections and Childhood Cancer case-control study conducted in Malawi (2005-2010). P falciparum status was determined by thick-film microscopy, malaria antigen (HRP2/pLDH) rapid diagnostic test, or PCR at enrollment. Malaria protective SNPs were extracted from genome-wide data measured using Infinium Omni5Exome-4 v1.3 BeadChip (Illumina, San Diego, CA, USA). Genotyping for α3.7-thalassemia deletions (/-α3.7)utilized droplet digital polymerase chain reaction. We tested for associations between BL and the malaria index SNPs, a genotypes, and P. falciparum positivity using generalized linear mixed models, controlling for age, sex, and the first 3 population-specific principal components as fixed effects and for genetic relatedness (calculated from genome-wide data) as a random effect. We considered a p-value < 0.05 for statistical significance for malaria index SNPs.
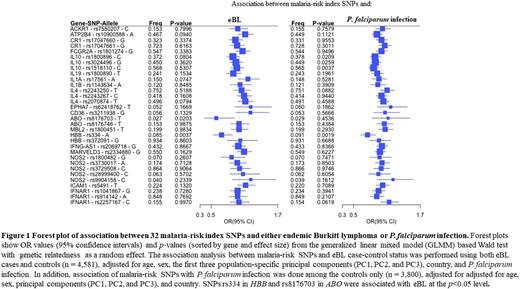
Results The mean age was similar for BL cases and controls (7.19 years versus 7.43 years, p= 0.07), but BL cases were more likely to be males (63.2% versus 52.1%, p£0.0001). P. falciparum positivity was lower in BL cases than controls (35.2% versus 48.3%, p<0.0001). Figure 1 shows the associations between 32 malaria index SNPs with BL and with P. falciparum positivity. Odds of BL were significantly decreased in presence of HBB rs334 (odds ratio [OR]= 0·687, 95% CI 0·533-0·885; p=0·0037) and ABO rs8176703 (0·591, 0·379-0·922; p=0.0203). Results for α-genotypes included the normal genotype (αα/αα), triplication (ααα/αα), -α3.7/αα, -α3.7/-α3.7deletions and unknown. Using αα/αα as the reference, the ORs for BL were 1.82, p=0.06 for ααα/αα; 1.07, p=0.469 for -α3.7/αα; and 0.78, p=0.15 for -α3.7/-α3.7. The odds of BL were significantly decreased with P. falciparum positivity in analyses without the two significant index SNPs (OR= 0.526, 95% CI 0.428-0.633, p=9.64x10-12) and changed minimally when the two SNPs were included (OR= 0·524, 0·435-0·630, p=7·291x10-12) .
Conclusion Based on 4,581 children in four high malaria burden countries, we found reduced odds of BL among carriers of malaria index variants in HBB and ABO genes which suggests that these variants may protect against BL as well. Additionally, our findings of decreased odds of BL with P. falciparum positivity at enrollment suggest premunition may be protective against BL, independent of the significant associations with SNPs in HBB and ABO. Together, our findings further enhance the evidence of a causal role of malaria in BL; coordination between malaria and BL programs in countries with high malaria/BL burden should be strengthened.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal