Abstract
INTRODUCTION Mantle Cell Lymphoma (MCL) is characterized by heterogenous disease courses ranging from indolent cases, that do not require therapy for years, to highly aggressive disease with a poor prognosis despite intensive treatment regimens. This emphasizes the need for accurate prognostic biomarkers to guide risk-adapted treatment strategies. The established risk factors used in the clinic today including high Mantle Cell Lymphoma International Prognostic Index (MIPI), high Ki67 proliferation index, high MIPI-c, TP53 mutations, and blastoid morphology, only partly identify patients in need of alternative treatment. Therefore, the search for clinically fit and biologically relevant biomarkers has been intensified. This has led to the proposal of two new NanoString-based, proliferation-associated prognostic biomarkers: the mRNA-based MCL35 score (Scott et al. 2017) and the circular RNA based circSCORE (Dahl et al. 2021). Both biomarkers have shown promising prognostic potential, but a comparison has never been done before. In this study we aimed at comparing MCL35 and circSCORE in 149 patients from the uniformly treated MCL2 and MCL3 cohorts.
MATERIALS AND METHODS
We included 149 patients from the Nordic patient cohorts MCL2 (70 patients) and MCL3 (79 patients) with sample data on both circSCORE and MCL35 along with long-term follow up data including progression free survival (PFS) and overall survival (OS). Gene expression analysis was obtained with the NanoString nCounter technology after RNA-isolation, using samples originating from both diagnostic lymph-nodes and non-nodal tissue. We also performed individual analyses on the MCL3 cohort only to eliminate the potential risk of overfitting, since the circSCORE is trained upon the MCL2 cohort.
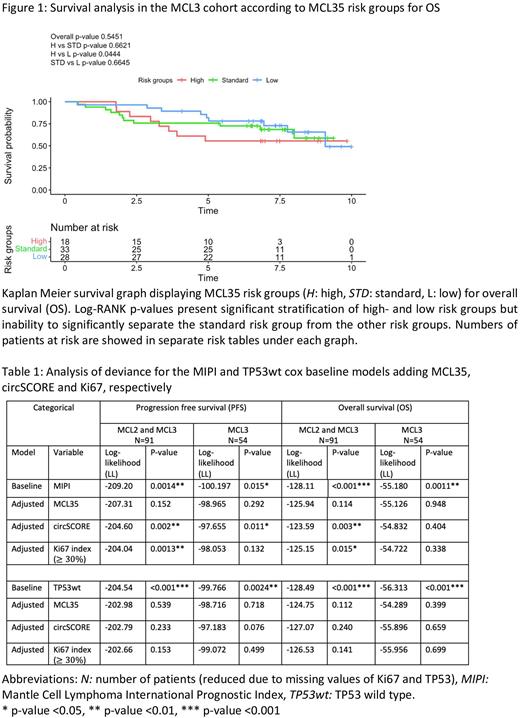
RESULTS We report that both circSCORE (PFS log-RANK p-value 0.0048 and OS log-RANK p-value 0.0426) and MCL35 (PFS log-RANK p-value 0.0422 and OS log-RANK p-value 0.0444) in the MCL3 only survival analyses displayed equal significant, prognostic potential in stratifying high and low risk groups. These analyses also revealed inability to significantly stratify the MCL35 standard risk group from the high- and low MCL35 risk groups (figure 1). Neither continuous circSCORE nor continuous MCL35 held prognostic significance when adjusting for known high-risk factors in multivariable cox regression, where only MIPI and TP53 mutation maintained significant results in all settings, except for PFS in MCL3 only (data not shown). The categorial data were analyzed with log-likelihood (LL) using the MIPI and TP53wt (wild type) cox models as baseline models (table 1). The results showed that both circSCORE and Ki67 added significant improvement to the MIPI baseline model in the pooled, MCL2+MCL3 setting, but most interestingly, only circSCORE added significant improvement in MCL3 only for PFS (LL -97.655 vs -100.197, p-value 0.011). Neither circSCORE, MCL35 nor Ki67 added significant improvement to the TP53wt baseline model (table 1).
CONCLUSION Both circSCORE and MCL35 demonstrated equal prognostic potential in separating high-and low risk groups in this study. Neither circSCORE, MCL35 nor Ki67 added significant improved value to the TP53wt baseline cox model, but circSCORE added significant improved value to the MIPI baseline cox model, even outperforming Ki67. This implies a new combined prognostic biomarker "MIPI-circSCORE", which could reflect the complexity of MCL through both clinical and biological, proliferation-associated features. However, the overall performance of the MCL35 risk score might be affected due to the inability of separating the standard risk group from the other MCL35 risk groups, the limited number of patients, and the tissue specific criteria of the MCL35 score not being met in this study. Therefore, the findings of this study should be further explored in larger MCL-cohorts to ensure stronger statistical power. Also, testing the biomarkers in MCL-relapse would be of great value to examine the prognostic potential in other settings of the heterogenic course of MCL. This is warranted to clarify the clinical future relevance of these biomarkers in supporting risk-adapted treatment regimens in patients with MCL.
Disclosures
Scott:AstraZeneca: Consultancy, Honoraria; Incyte: Consultancy; Roche: Research Funding; Janssen: Consultancy, Research Funding; NanoString: Patents & Royalties; Abbvie: Consultancy. Holte:Takeda: Honoraria, Other: Advisory board; Nordic Nanovector: Honoraria, Other: Safety committee; Novartis: Honoraria, Other: Advisory board; Gilead: Honoraria, Other: Advisory board; Genmab: Honoraria, Other: Safety committee; Incyte: Honoraria, Other: Advisory board. Jerkeman:Novartis: Honoraria; Orion: Honoraria; Incyte: Honoraria; Kite/Gilead: Consultancy, Honoraria, Research Funding; Genmab: Honoraria; AstraZeneca: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding. Grønbæk:Janssen: Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; Nanexa: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal