Abstract
Background: Over one-third of older adults with AML do not receive chemotherapy because of fear of toxicities and functional decline. This highlights the critical and urgent need to generate knowledge of functional changes following newer treatments.
Methods: As a part of a pragmatic single-center phase II investigator-initiated trial (ClinicalTrials.gov ID: NCT03226418), we enrolled older adults (≥60 years) with a new diagnosis of AML. We repeated geriatric assessment (GA) and health-related quality of life (HRQOL) before and 3 months after chemotherapy initiation. GA included measures of physical function (e.g. short physical performance battery or SPPB), Montreal Cognitive Assessment (MoCA), Hematopoietic Cell Transplantation Comorbidity Index, Patient Health Questionnaire-9 (PHQ-9), Mini Nutritional Assessment-Short Form. Frailty was defined as impairment in 2 or more domains on geriatric assessment (excluding MoCA). HRQOL was measured using European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C-30 (v 3.0). Established cutoffs were used to determine a clinically meaningful change in various measures from baseline to 3 months.
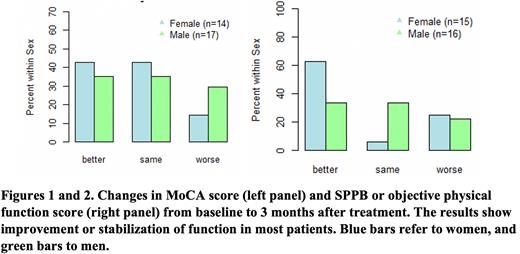
Results: 59 eligible patients were enrolled and had median age of 69 years and median KPS of 80. Patients received treatment with predominantly low-intensity chemotherapy (hypomethylating agent +/-venetoclax or midostaurin); six patients received intensive chemotherapy. 56% of patients had ≥2 comorbid conditions, and 69% each had impaired MoCA scores and impaired objective physical function at baseline. Whereas 29% had an established diagnosis of depression or anxiety, 66% had abnormal PHQ-9 with a score of 5 or more. 61% had impaired nutritional screen. A total of 43 patients completed some or all of the optional follow-up assessments at 3 months. Cognitive function or MoCA scores improved (64.5%) or stabilized (9.6%) from baseline to 3 months after treatment (Figure 1). We also noted improvement (51.6%) or stabilization (22.6%) in objective physical function (Figure 2). PHQ-9 scores showed improvement or stabilization in about 90% of patients. Nutritional scores showed variable changes with 40% demonstrating improvement. On HRQOL assessment, global health status score, and role functioning moderately improved from baseline to three-month after enrollment. Longitudinal cognitive function over 3 months differed based on the presence of frailty and multimorbidity (≥2 comorbid conditions) at baseline. Improvement in MoCA scores was noted in 31.8% (95% CI 16.4-52.7%) and 50.0% (95% CI 23.7-76.3%) of patients with versus without baseline frailty. The risk of worsening of cognitive function was 31.5% (95% CI 15.4-54.0%) for patients with multimorbidity at baseline versus 16.7% (95% CI 4.7-44.8%) for those without.
Conclusion: We demonstrated an overall improvement or stabilization of functional status and HRQOL in a high proportion of older adults with AML, despite high prevalence of functional impairments and significant comorbidity burden at baseline. These results should encourage oncologists to offer chemotherapy even to older adults with multiple comorbid conditions or poor functional status at the time of diagnosis of AML.
Disclosures
Bhatt:Chimerix: Other: Drug support for a trial; Pfizer: Research Funding; Servier Pharmaceuticals: Honoraria; Jazz: Research Funding; National Marrow Donor Program: Research Funding; Protagonist: Other: Member, Safety Monitoring Committee ; Genentech: Honoraria; Incyte: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding. Al-Kadhimi:Regeneron: Current equity holder in private company; Pacific: Current equity holder in private company; Moderna: Current equity holder in private company; Intellia: Current equity holder in private company. Armitage:Cardiff Oncology: Membership on an entity's Board of Directors or advisory committees. Holstein:Sanofi: Consultancy; Oncopeptides: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; BMS/Celgene: Consultancy, Research Funding; GSK: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Secura Bio: Consultancy; Genentech: Consultancy. Gundabolu:BioMarin Pharmaceuticals: Honoraria; BMS: Honoraria; Novartis: Honoraria; Blueprint Medicines: Honoraria; Samus: Research Funding; Jazz: Honoraria; Pfizer: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal