Abstract
The microRNA 15a/16-1 cluster on chromosome 13 is commonly lost in chronic lymphocytic leukemia (CLL). Even though this deletion is considered to be a CLL-associated genomic aberration, loss of miR-15/16 occurs in 79% of primary acute myeloid leukemia (AML) and in patients with myelodysplastic syndrome (MDS) that transformed into AML. A second highly homologous locus, miR-15b/16-2, is present in both human and mice, and deletion of either miR-15/16 cluster in mice leads to CLL. Unexpectedly, when both loci are deleted, only 23% of miR-15/16 double knock out (DKO) mice develop various non-Hodgkin's lymphomas, whereas 77% develop AML. Consistent with this, DKO mice have an expanded Gr-1+CD11b+ granulocytic population in both bone marrow (BM) and spleen. These observations led us to hypothesize that the miR-15/16 clusters can act coordinately to regulate lineage determination and proliferation in early hematopoiesis. Their loss might influence leukemogenesis in CLL and AML by altering the number and function of hematopoietic stem cells (HSCs) and/or their downstream lineages and potentially increasing a population of preleukemic stem cells.
To test this, using flow cytometry, we analyzed the numbers and frequency of HSCs and progenitors in DKO and C57BL/6 (B6) mice. We discovered major changes in HSCs and the common myeloid progenitors (CMP) but similar numbers of common lymphoid progenitors. Therefore, we focused on the myeloid lineage, finding that CD16/32-CD34- megakaryocyte/erythroid progenitors (MEP) were significantly increased in the Lin- cKit+ compartment of DKO mice (44.9% vs 29.8% P = 0.01), but CD16/32+CD34+ granulocyte-macrophage progenitors (GMP) were decreased (19.1% vs 40.1% P = 0.01). This was surprising since we also found that a GMP progeny population, CD11b+Gr-1+ cells, was enriched in DKO compared to B6 BM (60-70% vs 40%). To determine if this was a result of a differentiation defect in an earlier progenitor, we assessed the frequency of Lin-Sca1+cKit+ (LSK) populations, finding that DKO mice had significantly more CD150+CD48-CD34- LT-HSCs among LSK cells (14.3% vs 7.8% P = 0.03), and fewer CD150-CD48+ multipotent progenitors (MPP) (58.5% vs 68.6% P = 0.04). Of note, there was an increased absolute count for all progenitors analyzed, possibly due to an increase in BM cells and/or disproportional increases of certain cell types.
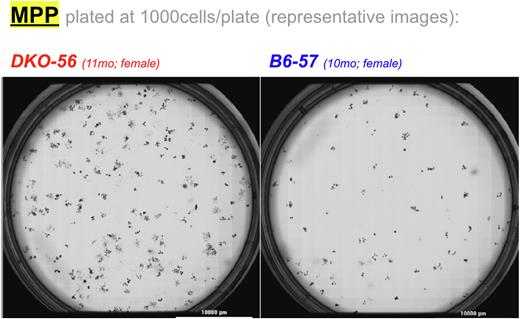
Since the observed myeloproliferative phenotype could be due to 1) an absolute increase of progenitor cells; 2) malfunctioning progenitor cells that aberrantly differentiate and proliferate; 3) or both, we tested whether DKO LSK cells had distinct functional characteristics in vitro. Methylcellulose replating of LT-HSC and MPP in the presence of SCF, IL-3, and IL-6 indicated that DKO LT-HSC gave rise to consistently fewer colonies as compared to B6 LT-HSC (21.5 vs 37.2 colonies/100 cells plated P = 0.02). Unexpectedly, we found the opposite for MPP, as DKO MPP consistently exhibited higher colony-forming activity than B6 MPP (16.6 vs 5.1 colonies/100 cells plated P = 0.04) over the course of serial replating experiments. This was surprising in light of the significant decrease in MPP frequency we had observed by immunophenotyping. Furthermore, B6 MPP cells ceased forming colonies after 3 rounds of replating, whereas DKO MPP continued for 5 rounds, suggesting that the absence of miR-15/16 increased the self-renewal potential of the CD150-CD48+ MPP cells. This was further supported by immunophenotyping analysis showing increased LSK cells derived from DKO MPP colonies (25.8% vs 4.4% P = 0.03). In concordance with the initial observation, an increase in DKO MPP colony-derived CD11b+Gr-1+ cells was at least partly responsible for the myeloproliferative defect in the mature cell compartment (76.1% vs 56.9%P = 0.01). Together, these results suggest that in miR-15/16 DKO mice, the phenotypically defined MPP compartment contains leukemia-initiating cells.
Overall, our findings reveal a novel role for the miR-15/16 clusters at the top of the hematopoietic hierarchy. Future experiments focusing on these DKO progenitors that appears to be endowed with leukemic stem cell potential may provide clues to understand how the targets of miR-15a, miR-15b and miR-16 orchestrate their effects on lineage commitment and how their absence may lead to initiation of AML and CLL.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal