Abstract
Acute myeloid leukemia (AML) is an aggressive myeloid malignancy with high relapse (40-60%) and low median survival rate (5-7 months post treatment in relapse setting), particularly for patients over 65 years old. Genomic investigations of AML have identified recurrent mutations, such as FMS-like tyrosine kinase 3 (FLT3, 20-25%) and Nucleophosmin (NPM1, 30%). When mutated, FLT3 acquires an internal tandem duplication (FLT3-ITD) and causes aberrant STAT5/AKT signaling. For NPM1, mutation frequently occurs at nuclear export signal, which results in cytoplasmic localization (NPM1c). In AML, these driver mutations co-occur with other genetic lesions, particularly in epigenetic regulators. Clincally, patients with FLT3-ITD mutations have a very poor prognosis. While the pathophysiology is not completely understood, direct inhibition of FLT3 by tyrosine kinase inhibitors have, to-date, been underwhelming. In contrast, the NPM1c mutation in AML patients is favorable and chemotherapy sensitive when NPM1c is the sole driver mutation. Yet, co-mutation of NPM1c with other known AML mutations, such as Flt3-ITD, negates this favorable prognosis.
STAG2 (Stromal Antigen 2) is a member of cohesin complex that is recurrently mutated in >10 cancers and is essential in maintaining the integrity of the 3-dimensional genome partitioning structure known as topologically structural domains (TADs). Previously, our work has demonstrated that depletion of various cohesin factors, including Stag2, leads to increased hematopoietic stem and progenitor cell (HSPC) self-renewal and myeloid-biased differentiation. Loss of Stag2 leads to impairment of sub-TADs and affects the capability of key hematopoietic transcription factors, such as PU.1, to access and engage their target genes. In the context of AML, disruption of TADs can lead to altered transcriptional output and cellular behavior. Mutation of STAG2 frequently arises in AML (16%) and co-occurs with NPM1 and FLT3 oncogenes.
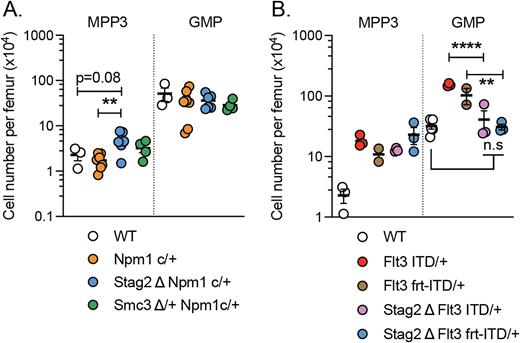
To examine the consequences of co-occurring Stag2/Npm1c or Stag2/Flt3-ITD mutations, we generated two compound mutant murine models crossed to conditional alleles of Stag2, Npm1c and constitutively active Flt3-ITD in the Ubc-CreER background. At 6-8 weeks of age, tamoxifen was administered to mice harboring Stag2/Npm1c or Stag2/Flt3-ITD mutations to delete Stag2 or activate Npm1c alleles respectively, while the Flt3-ITD mutation is active since birth. Immunophenotyping analysis was performed at 4 weeks post mutation activation in the bone marrow and peripheral blood. For mice carrying double Stag2/Npm1c mutation, there was no change in the number of stem cell population (HSCLT). In contrast, comparing to Npm1c/+ mice, Stag2-/-Npm1c/+ mice had an elevated myeloid-biased multipotent progenitor population (MPP3, LSK flk2- CD150- CD48+). However, there was no change in granulocyte-monocyte progenitor (GMP) number (Figure 1A). To determine if the myeloid bias was due to lack of the entire cohesin complex, we generated the Smc3/Npm1c mutant mice, where both Stag1-cohesin and Stag2-cohesin complexes were partially depleted upon heterozygous deletion of Smc3. Interestingly, there were no changes in MPP3 population in Smc3+/-Npm1c/+ mice, pointing to a Stag2-cohesin specific phenomenon. In the Stag2/Flt3-ITD co-mutation model, loss of Stag2-cohesin resulted in no changes in MPP3 cells compared to monogenic Flt3-ITD mutation. However, we did observe a normalization of GMPs, suggesting that Stag2-cohesin loss is crucial for abnormal myeloid differentiation in Flt3-ITD background (Figure 1B).
In order to evaluate whether the order of mutations impacted immunophenotypic aberrancy, we additionally generated a stepwise Stag2/Flt3-ITD mutational model by deleting Stag2 first (via Mx1-Cre) for 4 weeks followed by tamoxifen-inducible Frt activated Flt3-ITD mutation (Flt3 frt-ITD/+) for 4 weeks. Interestingly, there was no difference in the normalization effect between constitutive and conditional activation of Flt3-ITD mutation, suggesting the loss of Stag2-cohesin is not required prior to Flt3-ITD acquisition. Further characterization of these co-mutational murine models is in progress. With these models, we aim to uncover the dependency of Stag2-cohesin in leukemia maintenance.
Disclosures
Bowman:Mission Bio: Honoraria, Speakers Bureau. Viny:Arima Genomics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Nooma Bio: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal