Abstract
BACKGROUND: Hemophagocytic lymphohistiocytosis (HLH) is a rare and life-threatening syndrome of excessive immune activation. The disease is associated with a massive systemic inflammatory response requiring immediate and aggressive treatment. Secondary HLH (sHLH) is triggered by malignancy, autoimmune disease, or infection and believed to be caused by uncontrolled activation of immune cells, particularly monocytes/macrophages and CD8+ T cells. Currently there are no approved therapies for sHLH.
Signal regulatory proteins (SIRPs) are cell surface receptors expressed on cells of the myeloid lineage and T cells. ELA026 is a fully human, monoclonal immunoglobulin G1 (IgG1) SIRP-directed antibody that binds to and marks for destruction SIRP-expressing cells. By reducing myeloid-derived antigen presenting cells and interferon gamma-producing CD8+ T cells, ELA026 has the potential to halt the initiation and progression of the inflammatory process in sHLH. A proof-of-concept Phase 1b trial for assessing ELA026 efficacy in sHLH is currently underway (ClinicalTrials.gov Identifier: NCT05416307).
OBJECTIVES: To characterize the molecular pharmacology of ELA026 and to evaluate its pharmacokinetic (PK) profile and pharmacodynamic (PD) effects in non-human primates (NHP).
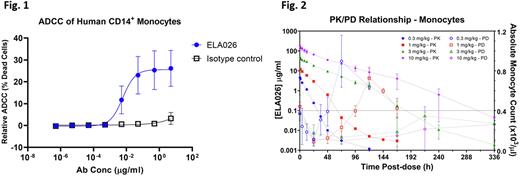
RESULTS: ELA026 was identified from a hybridoma library generated from a traditional murine immunization campaign and selected for its binding to SIRPs. ELA026 does not inhibit binding of CD47 to SIRPs and does not interfere with SIRP signaling. In vitro studies with ELA026 have demonstrated that compared to humans, the cynomolgus monkey (Macaca fascicularis) is a pharmacologically-relevant species with comparable cellular SIRP expression, demonstrates similar ELA026 affinity to homologous SIRP isoforms, and has equivalent ELA026-induced antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) potency of SIRP+ primary monocytes. ELA026 also induces ADCC of human SIRP+ T lymphocytes. In in vitro human cell systems, ELA026's effective concentration for inducing 90% maximal (EC90) ADCC (Fig. 1) and ADCP was ~0.1 µg/mL. In vivo, intravenous (IV) administration of ELA026 to NHP demonstrated reproducible and dose-dependent PD including a rapid (<24 hrs) and significant reduction of circulating monocyte, granulocyte, and T cell counts (60-80% depending on cell type; monocytes shown in Fig. 2). Duration of PD effect was observed to be dose-dependent, where higher doses lead to increased durability of cellular depletion. A threshold of ~0.1 µg/mL was determined to be the minimum concentration necessary to establish and maintain PD effect, consistent with the effective concentrations for ADCC and ADCP determined in vitro. Reconstitution of all affected cell types to pre-dose levels is observed within days of ELA026 washout (i.e. - dropping below ~0.1 µg/mL circulating plasma concentrations), suggesting that bone marrow hematopoiesis is unaffected by ELA026. Duration of effect was impacted by high levels of anti-drug antibodies in the cynomolgus monkey, though this did not affect interpretation of the ELA026 concentration-effect relationship.
CONCLUSION: In vitro pharmacological studies demonstrate that ELA026 binds to SIRP proteins on the surface of primary human monocytes and T cells, inducing potent ADCC and ADCP. Consistent with these in vitro observations, in vivo administration of ELA026 in cynomolgus macaques shows a rapid and potent depletion of SIRP expressing monocytes and lymphocytes with a well-defined PK/PD relationship consistent with in vitro pharmacological results identifying ELA026 mechanisms of action. Reversibility of the PD effect was achieved following washout suggesting that ELA026 treatment is not associated with long-term immunosuppression observed with other similar cell depleting agents. These results support the clinical development of ELA026 for the treatment of myeloid and T lymphocyte driven disorders including sHLH.
Disclosures: All authors are employees and stake holders of Electra Therapeutics, Inc.
Disclosures
Rose:Electra Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Moore:Star Therapeutics: Current Employment; Electra Therapeutics: Current Employment. Cai:Electra Therapeutics: Current Employment, Current holder of stock options in a privately-held company. He:Electra Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Huck:Star Therapeutics: Current Employment; Electra Therapeutics: Current Employment. Razo:Electra Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Wong:Electra Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Chan:Electra Therapeutics: Current Employment; Star Therapeutics: Current Employment. Horvath:Electra Therapeutics: Current Employment; Star Therapeutics: Current Employment; Bluebird bio: Current Employment. Liu:Electra Therapeutics: Current Employment; Star Therapeutics: Current Employment. Panicker:Star Therapeutics: Current Employment; Electra Therapeutics: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal