Abstract
Introduction: L-glutamine (Endari®) is an amino acid approved for the treatment of sickle cell disease (SCD). The mechanism of action of L-glutamine in SCD is not fully understood but believed to involve, at least partly, augmentation of the de novo synthesis of intracellular glutathione, which can protect sickle erythrocytes from oxidant injury. As an endogenous compound, the pharmacokinetics (PK) of L-glutamine have not been extensively studied, and currently recommended dosing is based on single-dose studies. It is also unclear how physiology, food intake, and dose impact L-glutamine drug exposure in patients with SCD. Our objective was to understand the PK of multiple dose L-glutamine therapy using a population PK analysis to identify factors that may inform optimal dosing of L-glutamine in children and adults with SCD.
Methods: A total of 400 L-glutamine plasma concentration-time data points collected from 12 participants (NCT04684381) were available for population PK analysis. Each participant received 3 ascending oral doses (0.1 and 0.3 g/kg given BID and 0.6 g/kg given once daily) during a four-week study. Plasma L-glutamine and other amino acid levels were obtained before and after oral L-glutamine doses at each visit and quantified by ion exchange chromatography. Population PK analysis was performed using nonlinear mixed effect modeling by NONMEM 7.4 (ICON, Ellicott City, MD, USA). Multiple base model structures including one-compartment and two-compartment models were explored to describe the L-glutamine PK. In addition to the primary PK parameters clearance (CL/F) and volume of distribution (V/F), a relative bioavailability (F) was included in the modeling to account for the potential impact of dose and food on L-glutamine PK. Patient demographics and laboratory data, including body weight, body surface area, age, serum creatinine levels, urine creatinine levels, baseline glutamine level, meal intake, and dosages were evaluated as potential covariates using the stepwise selection method. The change in the objective function value (OFV) between two nested models was assumed to follow the χ2 distribution and was tested at a significance level of <0.01 (-6.64 points in OFV). The final model was evaluated by goodness-of-fit (GOF) plots and visual predictive check (VPC) analysis. For the VPC analysis, one thousand replicates of simulated datasets were generated using the final model, and the distribution of simulated observations was compared with the actual observations.
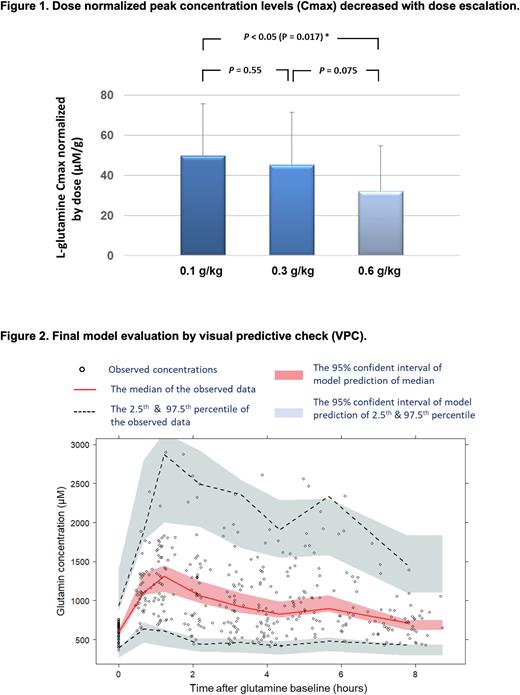
Results: A one-compartment with first-order absorption model best described the oral dose L-glutamine concentration-time profiles. The data did not support the inclusion of a lag time in drug absorption, which is consistent with the observed rapid absorption. In accordance with allometric theory, body size was found to be significantly correlated with glutamine oral clearance (CL/F; where F represents bioavailability, which is not measured and therefore unknown) and volume of distribution (V/F). We therefore used allometrically scaled body weight to account for differences in body size. Dose-normalized peak concentration (Cmax) decreased with dose escalation (Figure 1), indicating capacity-limited, non-linear PK of oral L-glutamine. Covariate analysis identified that baseline glutamine levels and dosages negatively correlated with glutamine clearance, whereas food intake did not significantly impact glutamine clearance. Higher doses decreased the relative bioavailability of oral glutamine, again indicating a non-linear saturable absorption. Model evaluation indicated no systemic bias (Figure 2). The estimates of L-glutamine oral clearance and volume of distribution were 66 L/h/70 kg and 140 L/70 kg, respectively.
Conclusions: This is the first report of a population PK analysis of L-glutamine in patients with SCD. We identified that higher baseline glutamine levels and higher doses were associated with lower L-glutamine clearance. In addition, increased doses reduced relative bioavailability, indicating that higher doses (>0.3 g/kg) may not provide higher drug concentrations. Food intake did not have a significant influence on glutamine clearance, implying that L-glutamine can be taken with or without food. Our findings will help to inform optimized dosing of L-glutamine for patients with SCD and potentially permit individualized PK-guided dosing to maximize treatment benefits.
Disclosures
Sadaf:Emmaus Medical: Research Funding. Ware:BMS: Research Funding; Addmedica: Research Funding; Hemex Health: Research Funding; Nova Laboratories: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: DSMB Chair; Editas: Other: DSMB Chair. Quinn:Dispersol Technologies: Honoraria; Emmaus Medical: Research Funding; Aruvant: Research Funding; FORMA Therapeutics: Honoraria; Novo Nordisk: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal