Abstract
CGD is an immunodeficiency characterized by infections and inflammation, particularly inflammatory bowel disease (IBD) and lung inflammation, resulting from mutations in genes encoding any one of 5 subunits of the phagocyte NADPH oxidase impairing production of microbicidal oxidants. Bone marrow transplantation (BMT) has been shown to be curative of both the predilection for CGD-specific fungal and bacterial infections, and CGD related IBD, and is being offered more frequently to patients with appropriate donors, particularly in younger children with matched related donors. However, older adult patients with CGD, especially those with fungal infection not responding to conventional therapy, those with very severe IBD, and particularly those with inflammatory lung disease characterized by significant hypoxia are often considered ineligible for transplant by many US BMT centers. Furthermore, graft versus host disease (GvHD) and graft rejection following BMT of CGD patients going into the transplant with significant inflammation disease has been a significant problem. We report here the results to date of our ongoing protocol using Campath (1mg/kg divided over 4d), Busulfan (5mg/kg divided over 2d) and TBI (300cGy) combined with post-transplant cyclophosphamide (100mg/kg divided over 2d) and sirolimus for patients with CGD, with outcomes that appear to address these issues. Patients with C-reactive protein >100 were excluded from the standard criteria protocol because this had been shown to be a high mortality risk. However 6 patients whose CRP exceeded this exclusion criteria received exceptions to be treated 'off protocol' because of infections not responding to conventional therapy.
Results: Number of patients to date: 30 plus 6 of whom were exceptions treated off protocol, and 1 retransplant; Follow up: 6 months to 5 years
Donor types: 34 MUD, 3 MSD
Patient Characteristics:
Age: Mean 30/Range 6 to 57,
Gender: 5F, 33M
CGD type: GP91 (29, incl.1 lyonized female carrier), P47 (6), P67 (1), P22 (1)
Transplant indications (some had more than one): Hypoxic inflammatory pulmonary disease (7), Colitis (11), Active infection (predominantly fungal) despite prolonged conventional therapy (14).
See table
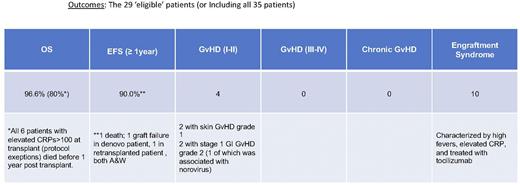
Summary: Low dose Busulfan with TBI plus Campath and post-transplant cytoxan followed by sirolimus for BMT of CGD patients with CRP <100 at transplant is well tolerated, and results in improved engraftment rates with minimal GvHD. Patients with severe infections, hypoxic inflammatory lung disease, severe colitis and older age, but who have CRP<100 tolerate this conditioning regimen and benefit from the transplant with resolution of their underlying infection and/or non-infectious lung disease and/or severe IBD, even in patients who would not be considered eligible for transplant at other centers. This NIH non-myeloablative /immune suppressive BMT conditioning regimen not only significantly reduces incidence of graft failure and severe GVHD, but expands the group of CGD patients eligible for transplant to older patients and those with ongoing infection, hypoxic inflammatory lung disease, and/or severe IBD. These patients can be transplanted successfully and achieve cure of infection and/or significant improvement in their inflammatory pulmonary disease and IBD if a matched donor is available with this NIH CGD BMT conditioning regimen. Our studies confirm that patients with CRP>100 at transplant should not be transplanted and going forward are excluded without exception from this protocol. Also of note is that transplanted CGD patients have a high rate of fever, diarrhea and newly elevated CRP, but without hypoxia or hypotension, appearing to be a form of 'engraftment syndrome' that occurs starting at 10-12 days post transplant and resolving about the time that ANC >200-300. In 10 patients this was sufficiently severe to warrant treatment with tocilizumab 4mg/kg, which in all cases promptly resolved the fever.
Disclosures
No relevant conflicts of interest to declare.
Author notes
∗Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal