Abstract
Background: EBV+ LMS is an ultra-rare, aggressive, and potentially fatal disease that develops in immunocompromised patients (Shannon-Lowe C and Rickinson A. Front Oncol. 2019). EBV+ LMS responds poorly to radiation and chemotherapy, resulting in limited treatment options and poor outcomes, with a median progression-free survival (PFS) under 3 months and a median overall survival (OS) under 12 months (Wang Z. Cancer Med. 2016). Thus, there is an urgent unmet need for effective therapies for patients with previously treated EBV+ LMS. Tabelecleucel is an investigational, off-the-shelf, allogeneic EBV-specific T-cell immunotherapy currently under study in patients with potentially fatal EBV+ diseases, including EBV+ LMS. We previously reported on 12 patients with EBV+ LMS treated with tabelecleucel. With a median follow-up of 22.6 months, we observed an objective response rate (ORR) of 16.7% (2 partial responses [PR]), clinical benefit rate (CBR) of 83.3% (2 PR and 8 stable disease [SD]), and median OS of 77.4 months (Kurlander LS et al. Ann Oncol. 2018), suggesting that tabelecleucel may provide clinical benefit to patients with EBV+ LMS. Here, we report updated efficacy and safety data from this disease cohort, including longer follow-up and an additional six patients.
Methods: Tabelecleucel was evaluated in two single-center, open-label studies (NCT00002663; NCT01498484) and a multicenter expanded access program (NCT02822495, which includes two non-overlapping protocols and six additional patients for this analysis). Tabelecleucel was given at 1.0-2.0 x 106 cells/kg/dose on days 1, 8, and 15 of every 4- to 6-week cycle. Efficacy endpoints included ORR (complete response [CR] and PR assessed by computed tomography-based RECIST 1.1), duration of response (DOR), CBR (CR, PR, and SD), PFS, and OS, with disease status assessed by investigators. Safety was assessed based on treatment-emergent serious adverse events (TESAEs) reported during treatment and follow-up.
Results: A total of 18 patients with EBV+ LMS received ≥1 dose of tabelecleucel. Median age was 8.9 years (range, 3-35) and 44.4% of patients were male. Among the 12 patients with available data, the median number of prior systemic therapies was one (range, 0-3); all 18 patients received at least one prior treatment, which may have included surgical resection.
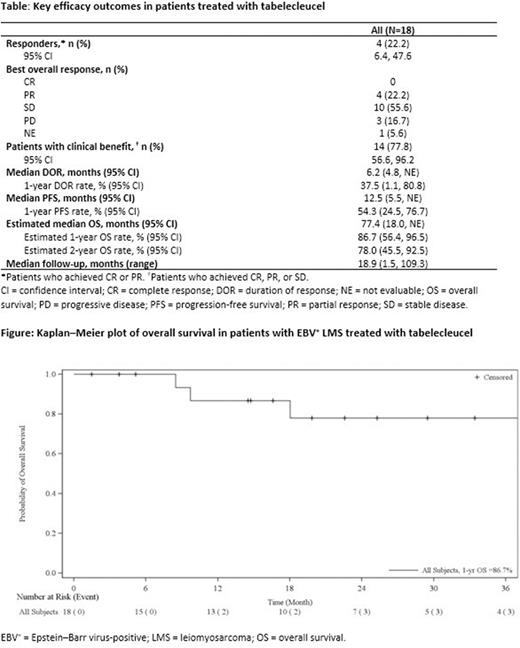
Median follow-up (95% confidence interval [CI]) for all patients was 18.9 months (1.5, 109.3). We observed a CBR (95% CI) of 77.8% (56.6, 96.2) and ORR (95% CI) of 22.2% (6.4, 47.6; PR in all cases) [Table]. Median PFS (95% CI) was 12.5 months (5.5, not evaluable [NE]) with a 1-year PFS rate (95% CI) of 54.3% (24.5, 76.7). Median DOR (95% CI) was 6.2 months (4.8, NE) with a 1-year DOR rate (95% CI) of 37.5% (1.1, 80.8). The estimated median OS (95% CI) was 77.4 months (18.0, NE) (Table; Figure). The estimated 1-year survival rate was 86.7%, and the estimated 2-year survival rate was 78.0% (Table).
Overall, TESAEs were reported in 10 (55.6%) patients. TESAEs were grade ≥3 in 8 (44.4%) patients, and there was one fatality (5.6%, disease progression). None of the reported TESAEs were considered related to study treatment. Overall, the safety profile was consistent with previously reported data (Prockop SE et al. Blood. 2017; Kurlander LS et al. Ann Oncol. 2018; Prockop SE et al. J Clin Invest. 2020), with no reports of tumor flare reaction, cytokine release syndrome, transmission of infectious diseases, graft-versus-host disease, or infusion reaction related to tabelecleucel.
Conclusions: This updated analysis of 18 patients with previously treated EBV+ LMS from two studies and a multicenter expanded access program, which includes six additional patients and longer follow-up, demonstrates that tabelecleucel may provide clinical benefit and a favorable safety profile for patients with this ultra-rare disease. These promising results support the further study of safety and efficacy of tabelecleucel in patients with EBV+ sarcomas as part of an ongoing Phase 2 multicohort study (NCT04554914).
Disclosures
Doubrovina:Atara Biotherapeutics: Patents & Royalties. Dinavahi:Atara Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Gamelin:Atara Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Ghobadi:Celgene: Consultancy; CRISPR Therapeutics: Consultancy; Amgen: Consultancy, Research Funding; Atara: Consultancy; Wugen Inc: Consultancy; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Genentech: Research Funding. Mascarenhas:Amgen: Consultancy; Jazz, Eli Lilly, Pfizer, Incyte, ER Squibb, BMS: Other: Research funding to institution, no personal remuneration, Research Funding; AstraZeneca, Salarius, Turning Point, Bayer: Other: Research funding to institution, no personal remuneration, Research Funding. Nikiforow:Kite/Gilead: Consultancy; Iovance: Consultancy; GlaxoSmithKline: Consultancy. O'Reilly:Atara Biotherapeutics: Consultancy, Patents & Royalties: Received royalties following licensure of the EBV-specific T-cell bank by Atara Biotherapeutics and has subsequently received research support and consultant fees from Atara Biotherapeutics, Research Funding. Shulkin:Innervate Radiopharmaceuticals, LLC: Other: Provide blinded interpretations of nuclear medicine images. Wahlstrom:Atara Biotherapeutics: Consultancy, Current Employment, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months. Xing:Atara Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Prockop:Memorial Sloan Kettering Cancer Center (MSK): Other: Co-inventor of intellectual property transferred from Atara; AlloVir (through MSK): Research Funding; Atara Biotherapeutics (through MSK): Other: Co-inventor of IP licensed to Atara. Dr Prockop transferred IP rights to MSK & has no personal financial interests in Atara. MSK has financial interests in Atara/IP interests related to this study, Research Funding; Jasper Therapeutics (through MSK): Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal