Abstract
Background: Despite multiple therapeutic options for patients with multiple myeloma (MM), clonal heterogeneity and acquired resistance lead to relapse and disease which is refractory to existing treatments in almost all patients. Patients with relapsed or refractory MM (RRMM) whose disease progresses after receiving standard therapies such as proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and anti-CD38 monoclonal antibodies (mAbs) are challenging to treat, and new, effective treatment options are needed. Belantamab mafodotin (belantamab) is approved as a monotherapy for patients with RRMM who have previously progressed after 4 prior lines of therapy (LOT), including a PI, an IMiD, and an anti-CD38 mAb; however, disease control remains suboptimal, with response rates of ~30% and a median progression-free survival (PFS) of 2.9 months. There is an unmet need for new, accessible and efficacious therapeutic options with different mechanisms of action that can provide deeper, more sustained responses. Talquetamab is a first-in-class, off-the-shelf, T-cell redirecting, bispecific antibody targeting both G protein-coupled receptor family C group 5 member D (GPRC5D) and CD3. In the phase 1/2 MonumenTAL-1 study (NCT03399799), talquetamab monotherapy had a manageable safety profile and showed encouraging efficacy in heavily pretreated patients with RRMM. MonumenTAL-1 was a single-arm trial and did not include a comparator, so there is a need for direct comparisons between talquetamab and other approved therapies in this heavily-pretreated patient population. The phase 3, multicenter, randomized, open-label, active-controlled MonumenTAL-5 trial will compare the efficacy and safety of talquetamab monotherapy with belantamab in patients with RRMM with ≥4 prior LOT.
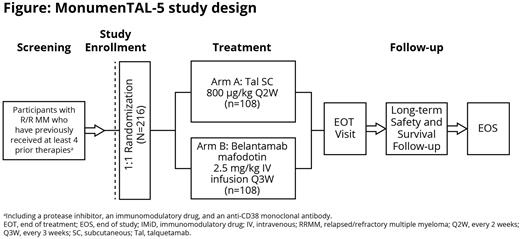
Study Design and Methods: Eligible patients include those ≥18 years of age with documented MM per International Myeloma Working Group (IMWG) criteria, measurable disease, Eastern Cooperative Oncology Group performance status 0-2, who received ≥4 prior LOTs (including at least one PI, one IMiD, and an anti-CD38 mAb), and have progressive disease on or after their last regimen. Patients with prior GPRC5D-directed treatment or belantamab will be excluded. Approximately 216 patients will be randomized 1:1 to receive 42-day cycles of subcutaneous talquetamab (0.8 mg/kg every other week) or intravenous belantamab (2.5 mg/kg every 3 weeks), stratified by International Staging System stage (I/II vs III), number of prior therapies (4 vs >4), and prior BCMA-targeted therapies (naive vs exposed). Step-up doses of talquetamab will be administered before the first full dose of talquetamab in Cycle 1. Patients will receive treatment until disease progression, start of subsequent antimyeloma therapy, death, intolerable toxicity, withdrawal of consent, or end of study, whichever occurs first. The dual primary endpoints are PFS and overall response rate (ORR); response will be assessed per 2016 IMWG criteria as adjudicated by an IRC. Secondary endpoints include very good partial response or better rate, complete response or better rate, overall survival (OS), PFS on next LOT (PFS2), patient-reported outcomes, and incidence and severity of adverse events (AEs). Exploratory endpoints include the rate of minimal residual disease negativity. AEs will be graded by Common Terminology Criteria for AEs v5.0, except for cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome, which will be graded by American Society for Transplantation and Cellular Therapy guidelines, and corneal AEs, which will be graded according to the Keratopathy and Visual Acuity scale. The study will end when ~160 OS events have been observed across both arms. The study will open for enrollment in October 2022. Results from this study will provide insights into the efficacy and safety of talquetamab monotherapy compared with belantamab in heavily pretreated patients with RRMM.
Disclosures
Kumar:AbbVie,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive,: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE,: Research Funding; MedImmune/Astra Zeneca,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis,: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee. Garfall:Janssen, Novartis, Tmunity, CRISPR Therapeutics: Research Funding; Janssen, GSK, Amgen, Legend: Consultancy; Janssen: Other: Independent data monitoring committee. Trudel:Karyopharm: Honoraria; Takeda: Honoraria; AstraZeneca: Honoraria; Janssen: Honoraria, Research Funding; Forus: Consultancy; Sanofi: Honoraria; Genentech: Research Funding; Pfizer: Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Research Funding. Ocio:Amgen, BMS/Celgene, GSK, Janssen, Karyopharm, Oncopeptides, Pfizer, Sanofi, Takeda: Consultancy; Takeda: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; BMS/Celgene: Consultancy, Honoraria; Janssen, Takeda: Speakers Bureau; Oncopeptides: Consultancy, Honoraria; Karyopharm: Consultancy; GSK: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Amgen, BMS/Celgene, GSK, Janssen, Oncopeptides, Pfizer, Sanofi, Takeda: Honoraria; GSK: Research Funding. Scott:Janssen: Current Employment; GSK: Ended employment in the past 24 months. Huang:Janssen Pharmaceuticals: Current Employment. Ma:Janssen: Current Employment. Olyslager:Janssen: Current Employment. Thakkar:Janssen: Current Employment. Pei:Janssen R&D: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Heuck:Janssen R&D: Current Employment, Current equity holder in publicly-traded company. Terpos:Sanofi: Honoraria, Research Funding; BMS: Honoraria; EUSA Pharma: Honoraria, Other: Travel expenses; Genesis: Honoraria, Research Funding; GSK: Honoraria, Research Funding; Novartis: Honoraria; Amgen: Honoraria, Other: Travel expenses, Research Funding; Janssen: Honoraria, Research Funding; Takeda: Honoraria, Other: Travel expenses, Research Funding.
OffLabel Disclosure:
at the time of abstract submission, talquetamab is being investigated for the treatment of multiple myeloma but is not yet not approved
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal