Abstract
Background Idiopathic cytopenia of undetermined significance (ICUS) is a condition of unexplained blood cytopenia not meeting the diagnostic criteria of myelodysplastic syndromes (MDS). Recently, the newer designation of clonal cytopenia of undetermined significance (CCUS) has been proposed to characterize patients with ICUS in the presence of one or more somatic mutations or copy number abnormalities. Clinically, CCUS has been associated with an increased risk of developing MDS; however, the mechanism and factors associated with evolution remain unclear. We sought to determine the molecular similarity of cytopenic cases with equivocal morphological dysplasia to bona fide MDS, as well as whether serial sequencing of ICUS and CCUS cases could identify factors that predict evolution to MDS.
Methods We compared the incidence and frequency of somatic variants in 48 genes recurrently mutated in myeloid neoplasms (MNs), using targeted Next-Generation Sequencing (NGS) of bone marrow genomic DNA from 193 individuals with confirmed or suspected MDS or MDS/myeloproliferative neoplasm (MDS/MPN), including sequential investigation for 28 individuals at the time of diagnosis and during follow-up.
Cytopenias qualifying patients as ICUS were based on WHO laboratory diagnostic criteria: hemoglobin levels <130g/L for males or <120 g/L for females; platelet count <150x109/L; and neutrophils <1.8x109/L. MDS patients were further subclassified as low risk (MDS-LR), including "Very Low” and "Low” International Prognostic Scoring System-Revised (IPSS-R) categories; intermediate risk (MDS-IR), including "Intermediate” category; or high risk (MDS-HR), including "High” and "Very High” risk categories.
Results NGS further facilitated the diagnosis of all suspicious cases, either as MN (21%), CCUS (34%), or ICUS (45%). We found that there was no significant difference in most measured clinical features between CCUS and MDS-LR. While we saw significantly increased hemoglobin and platelet counts in individuals with CCUS compared with MDS-HR (Mann-Whitney U test; p=0.003 and p=0.012, respectively), no significant differences in cell counts were seen between CCUS and the MDS-LR or MDS-IR subgroups (Table 1). There was also no difference in overall survival between CCUS and MDS-LR when adjusted for age and sex in multivariable models (HR=0.575; 95% CI=0.256-1.294; p=0.330), while MDS-HR was associated with worse overall survival when compared with CCUS (HR=2.759; 95% CI=1.274-5.975; p=0.010).
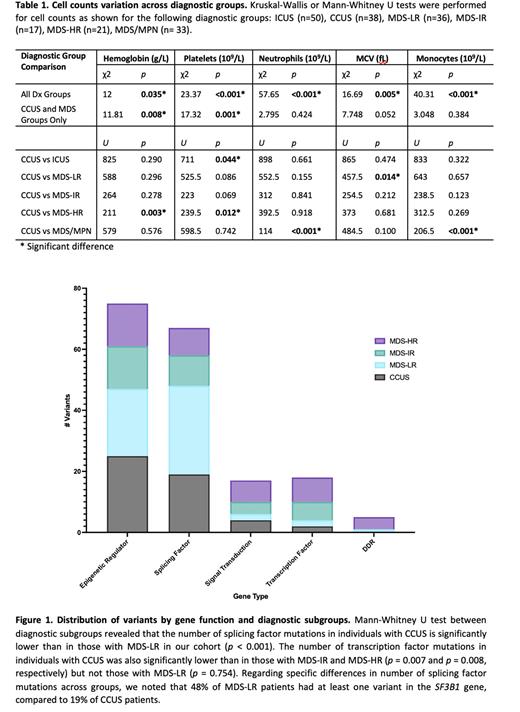
We saw no significant difference in clonal phenotypes, including number of variants or variant allele frequencies (VAFs), when comparing CCUS and MDS-LR. We further classified genes mutated in at least one patient in our cohort into functional groups for further analysis. These groups included epigenetic regulators: ASXL1, BCOR, DNMT3A, EZH2, IDH1, IDH2, KDM6A, PHF6, RAD21, STAG2, TET2; splicing factors: DDX41, SF3B1, SRSF2, U2AF1, ZRSR2; genes involved in signal transduction: BRAF, CALR, CBL, CSF3R, FLT3, GNB1, JAK2, KIT, KRAS, MPL, NF1, NPM1, NRAS, PTPN11, SETBP1; transcription factors: CEBPA, ETV6, GATA2, RUNX1; and genes involved in DNA damage repair: PPM1D and TP53. A significantly higher number of transcription factor mutations was seen when comparing CCUS to the MDS-IR and MDS-HR groups (p=0.007 and p=0.008, respectively), but not the MDS-LR group (p=0.754). We did, however, see significant significantly lower number of splicing factor mutations in CCUS cases compared with MDS-LR (p<0.001; Figure 1).
Serial sequencing revealed no significant associations between number, type, or VAF of variants initially present at ICUS or CCUS diagnosis compared to those later acquired at the time of MDS evolution. Of note, we saw an increased probability of evolution to MDS of individuals with CCUS compared to ICUS over the first 5 years (HR=3.569; 95% CI=1.029-12.380, p=0.045).
Conclusions Our analyses revealed no conclusive pattern associating clonal expansion or number of variants with evolution of CCUS to MDS, perhaps further supporting the similarity of these diseases and the proposal that CCUS should be classified as low-risk MDS in the next revision of the WHO guidelines. If future studies can better associate variants in specific types of genes with progression (such as splicing factors), this may also assist in predicting the probability of evolution and thus inform management strategies.
Disclosures
Buckstein:Takeda: Research Funding; BMS: Honoraria, Research Funding; Taiho: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal