Abstract
Background: Immune checkpoint inhibitors that target PD-1/PD-L1 are effective against some solid tumors and Hodgkin's lymphoma (HL). However, the therapeutic benefits of PD-1/PD-L1 inhibitors are limited to a small subset of patients with diffuse large B-cell lymphoma (DLBCL). As immune checkpoint monotherapies rarely elicit effective or long-term responses, targeting the PD-1/PD-L1/L2 pathway combined with other immunosuppressive signaling, such as CD73/A2aR adenosine signaling, has emerged as a promising strategy for cancer treatment. Here, we hypothesized PD-1/PD-L1/L2 and CD73/A2aR contribute to acquiring a CD8+ T cell dysfunctional phenotype. The genetic characteristics and clinical effectiveness of these two immune checkpoints need to be further investigated in DLBCL.
Materials and methods: We performed whole-exome sequencing/targeted deep sequencing to investigate the genetic characteristics of PD-1/PD-L1/L2 and CD73/A2aR. The immunosuppressive effect of these two pathways on the tumor microenvironment was evaluated via RNA sequencing. Single-cell RNA sequencing was further applied to investigate the dysfunctional CD8+ T cells. In addition, multiplex immunofluorescence staining was used to quantitatively assess the expression of dysfunctional CD8+ T cells in DLBCL.
Results: A frequent copy number gain of 9p24.1, previously described with the upregulation of PD-L1/L2 in HL, was identified in three patients with DLBCL (3/42; 7%), two of which had the non-germinal center B cell-like (GCB) subtype. No CNVs affecting CD73 or A2aR were identified.
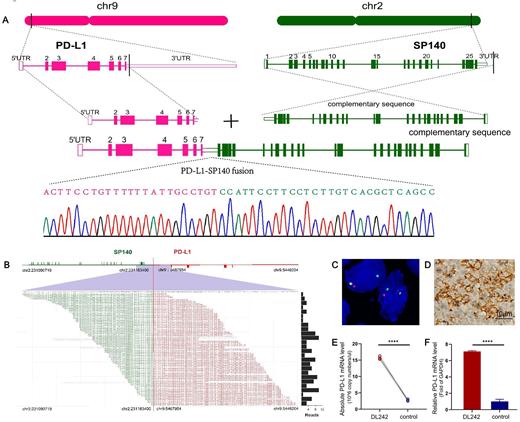
SP140 was identified as a novel translocation partner for PD-L1 and genomic breakpoint coordinates were mapped to chr9:5467954 and chr2:2311834 00 (NCBI Build 36.1) falling within the 3' untranslated region (UTR) of PD-L1 and the intergenic region downstream of SP140. This translocation partner lead to the upregulation of PD-L1 expression. Additionally, an in-frame PD-L1-PD-L2 inversion caused by the 3' portion of PD-L1 within intron 5 of PD-L1 fused with intron 3 of PD-L2 was also detected in DLBCL. This novel inversion was also ensured to result in the upregulation of PD-L1 expression. However, the expression of PD-L2 was downregulated.
Two nonsense mutations and one missense mutation were identified in PD-L1 in three DLBCL patients. The two nonsense variants, p.Arg125* and p.Gln173*, were predicted to generate truncated proteins lacking the transmembrane domain. Another variant, c.302 T>G was located in the coding region of the immunoglobulin subtype with PD-L1 residue isoleucine 101 mutated to serine (p.Ile101Ser). However, PD-L1 expression was not upregulated in these altered samples. Cytogenetic mutations that affected the PD-L1/L2 loci showed no difference in the distribution of subtypes. However, genetic translocations in the PD-L1/L2 loci were primarily associated with the non-GCB subtype of DLBCL.
Genetic mutations of CD73 were identified in four cases carrying two missense mutations. One mutation, c.185C>T, encoded the substitution of a highly conserved alanine into valine at position 62 (p.A62V), which is located in the functional calcineurin-like phosphoesterase domain of CD73. CD73 genetic mutations did not increase mRNA and protein expression. However, patients with genetically altered CD73 tended to have a better overall survival than patients with wild-type CD73.
Both PD-1/PD-L1 and CD73/A2aR signaling mediated the immunosuppressive microenvironment in DLBCL. The numbers of CD8+ T cells with PD-1 and A2aR expression were positively correlated with the number of dysfunctional CD8+ T cells (R2= 0.974, p = 0.013). According to the grades of dysfunctional CD8+ T cells we defined, grade 1 dysfunctional CD8+ T cells, with either PD-1+ or A2aR+, were significantly associated with poorer survival than grade 0 dysfunctional CD8+ T cells, with both PD-1- and A2aR-; and patients with grade 2 dysfunctional CD8+ T cells showed the worst clinical outcomes.
Conclusions: This study describes the additional genetic basis of PD-L1 overexpression and characterizes certain genetic alterations of CD73/A2aR in DLBCL. The degree of T cell dysfunction is correlated with clinical outcomes. Strategies that reverse T cell dysfunction by inhibiting PD-1/PD-L1/L2, particularly in combination with CD73/A2aR, may show potential as effective therapeutic options for DLBCL.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal