Abstract
Background: Children with refractory or relapsed (R/R) leukemia, particularly acute myeloid leukemia (AML), have limited curative options. Allogeneic hematopoietic stem cell transplantation (HSCT) for patients who achieve subsequent complete remission (CR) after relapse is generally required for long-term cure, but many children with multiply-R/R AML are unable to achieve CR with conventional salvage regimens. Dual therapy with the hypomethylating agent azacitidine and BCL-2 inhibitor venetoclax (aza/ven) is well-tolerated in adults with AML and has become the standard of care for elderly patients or those with co-morbid conditions precluding intensive chemotherapy. The safety of venetoclax in combination with cytarabine +/- idarubicin was established in children with R/R AML (Karol Lancet Oncology 2020), but pediatric experience with the aza/ven combination remains limited. We sought to describe our institutional experience with aza/ven-based therapy in children and adolescents/young adults with R/R acute leukemias.
Methods: We conducted a retrospective chart review of patients aged 0-21 years at initial leukemia diagnosis who were treated with commercially-available aza/ven at the Children's Hospital of Philadelphia (CHOP) from January 1, 2019 to June 30, 2022. Data were collected for baseline patient demographics, leukemia immunophenoptyping and genetics, therapy administration, clinical response, and post-aza/ven outcomes. Azacitidine 100 mg/m2 daily intravenously (IV) was given on days 1-5 of each 28-day cycle. Venetoclax was given daily as oral tablets with 3-day ramp-up dosing to minimize tumor lysis syndrome (TLS) risk as 200 mg (day 1), 400 mg (day 2), and 800 mg (days 3-28) adult equivalent dosing (Place Future Oncology 2018) with 50% dose reduction for patients receiving concomitant azoles.
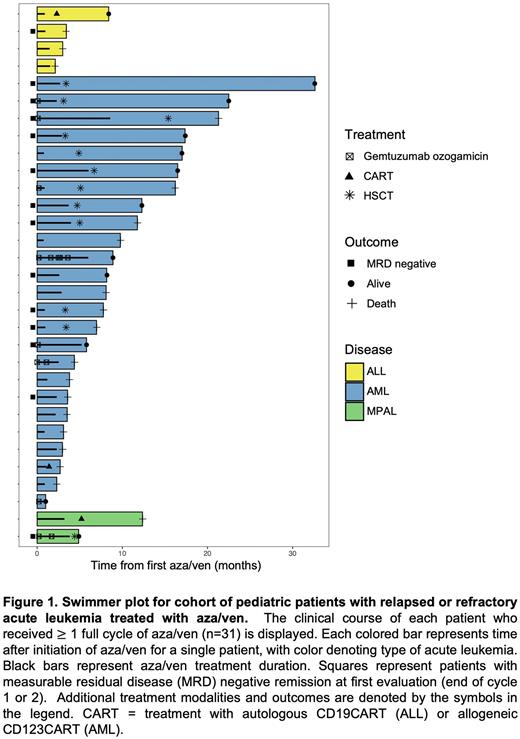
Results: Thirty-seven pediatric patients with R/R acute leukemia received aza/ven-based therapy at CHOP during the study period; 27 (72.9%) had AML, 7 (18.9%) had B-cell acute lymphoblastic leukemia (B-ALL), 1 had T-cell ALL (2.7%), and 2 (5.4%) had mixed phenotype acute leukemia (MPAL). Six children did not complete a full 28-day cycle of aza/ven due to progressive disease (n=4) or severe TLS (n=2). Within the cohort of patients who completed 1 cycle, 22/31 (71%) had multiply-relapsed leukemia, and 9/31 (29%) had primary chemorefractory disease. The median age at initial leukemia diagnosis was 8 years (range 0.5-21). Patients in this cohort received a median of 2 aza/ven cycles (range 1-6). Eight patients with AML also received one dose of gemtuzumab ozogamicin (GO) 3 mg/m2 IV (given on day 4, 5, or 8) with cycle 1 aza/ven; 3 patients received additional GO in subsequent cycles. Twelve of 31 patients who completed 1 cycle aza/ven had cycle 2 or later chemotherapy held or delayed, most commonly to facilitate resolution of drug-, infection- or leukemia-induced cytopenias. Amongst the 29 patients who underwent bone marrow response assessment after aza/ven cycle 1 (n=25) or cycle 2 (n=4), 14 were in CR with negative measurable residual disease (MRD) by flow cytometry (<0.1% for AML, <0.01% for ALL/MPAL), including young children with high-risk AML genetics such as CBFA2T3-GLIS2 and ETV6-EP300 fusions. One additional patient with residual leukemia after cycle 1 aza/ven achieved MRD-negative CR after cycle 2. The remaining patients had either persistent leukemia detectable in peripheral blood or were MRD+ in bone marrow at first response assessment.After receipt of aza/ven, 11 patients with AML and 1 with MPAL proceeded to HSCT, 2 patients with B-ALL were treated with autologous CD19 chimeric antigen receptor T cells (CD19CART), and 1 patient with AML received allogeneic CD123CART. The study cohort was followed for a median of 7.8 months (range 1-32 months). At last follow up, 39% (12/31) of patients were alive (Figure 1).
Conclusions: We report the experience of a single institution with 37 pediatric patients with multiply-R/R AML, ALL, and MPAL treated with aza/ven-based therapy, often in the outpatient clinic with good tolerability. Importantly, MRD-negative CR was achieved in 14/37 children (37.8%), nearly always after one cycle of aza/ven, which enabled consolidative HSCT in most responders and long-term survival of some patients. Formal clinical trial evaluation of aza/ven in children with R/R acute leukemias, ideally at an earlier stage of relapse, is warranted.
Disclosures
Tasian:Incyte Corporation: Research Funding; Kura Oncology: Consultancy, Research Funding; Beam Therapeutics: Research Funding; Syndax Pharmaceuticals: Consultancy; Aleta Biotherapeutics: Consultancy; GSK: Consultancy; bluebird bio: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal