Abstract
BACKGROUND: Despite excellent outcomes in pediatric leukemias, multiply relapsed or refractory patients have lower response rates to reinduction therapy and low overall long-term survival. (Hunger/Raetz, Blood 2020; Kaspers, Br J Haematol 2014) Clofarabine and Mitoxantrone have proven efficacy in children with leukemia and may display synergy together. (Jeha et al, J Clin Oncol 2009; Parker et al, Lancet 2010) Our previously reported Phase I results demonstrated the maximal tolerated dose of this combination to be Clofarabine 35mg/m2 x 5 days and Mitoxantrone 12mg/m2 x 4 days. Here, we present our final results utilizing this novel combination for high risk pediatric leukemias to achieve MRD negativity as a bridge to hematopoietic allogeneic stem cell transplantation (HSCT).
OBJECTIVE: We sought to determine the safety, overall response rate and long term EFS/OS in a Phase I/II trial of clofarabine in combination with mitoxantrone as reinduction therapy for relapsed or refractory pediatric acute leukemia.
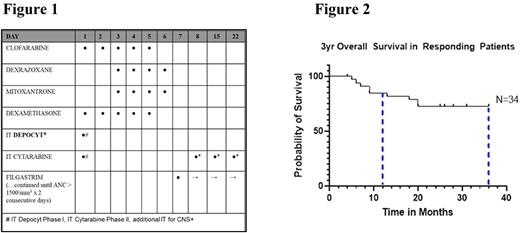
METHODS: We conducted a prospective, Phase I/II, dose escalation, safety and efficacy study (NCT01842672). Eligible patients were 0-30.99yr old with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML) either with relapse, induction failure or persistent post consolidation MRD. Patients were given 1 to 3 cycles of clofarabine (Phase I escalating doses 20, 30, 35 and 40mg/m2/day; RP2D 35mg/m2/day) Day 1-5, in combination with mitoxantrone 12mg/m2/day on Day 3-6. Figure 1. CNS prophylaxis was accomplished initially with intrathecal liposomal cytarabine and subsequently with standard cytarabine. MRD was defined by multidimensional flow cytometry as previously reported. (Loken et al, Blood 2012; Borowitz et al, Blood 2015)
RESULTS: A total of 39 patients enrolled (18 patients in Phase I, 21 patients in Phase II). Median Age was 13yrs (8months-23yrs). Demographics included 23 ALL (9 = IF/MRD, 11 = Relapse 1, 3 = Relapse 2), 16 AML (8 = IF/MRD, 6 = Relapse 1, 2 = Relapse 2). During the Phase I portion, there were 2 Grade III/IV toxicities at Dose Level 4 (1 hepatic toxicity, 1 prolonged myelosuppression) requiring de-escalation to Dose Level 3. Median time to neutrophil recovery was 24 days in both phases. The Phase I MTD (RP2D) of this combination was established at 35mg/m2/dose Clofarabine. In Phase II, one additional patient developed Grade IV prolonged myelosuppression. There were no other dose limiting toxicities. Thirty three of 39 (85%) leukemia patients achieved a CR after 1 cycle of therapy. This included 21 of 23 (91%) ALL patients and 12 of 16 (75%) AML patients. Of these, 88% achieved MRD negativity based on flow cytometry. Thirty one of 33 patients achieving CR went on to receive HSCT. One patient died prior to HSCT. Seven patients died of non-relapse HSCT complications. One patient died of recurrent disease post-HSCT. The remaining 24 patients continue to demonstrate complete remission with MRD negativity. The overall and event free survival at 1 year and 3 years for the cohort of patients who responded to therapy is 85% (CI95 0.84-0.97) and 77% (CI95 0.64-0.98), respectively at a median follow up time of 53 months (range 10-101). (Figure 2)
CONCLUSION: The combination of clofarabine and mitoxantrone reinduction therapy for relapsed or refractory acute pediatric leukemia has been demonstrated to be safe and well tolerated at a RP2D of 35mg/m2 Clofarabine in children with poor risk relapsed or refractory acute leukemias. Our response data is encouraging with 85% CR rate with high MRD negativity in leukemic patients allowing patients to safely proceed to allogeneic stem cell transplant with a 3yr EFS and OS of 77%. A larger cohort and longer term follow-up is needed to assess late toxicities, particularly cardiac, as well as EFS/OS more definitively at later endpoints and to better define subgroups. A successor trial is planned and ongoing.
Disclosures
Oesterheld:Servier: Speakers Bureau; Ymabs: Membership on an entity's Board of Directors or advisory committees. Loken:Hematologics Inc.: Current Employment, Current equity holder in private company. Cairo:Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Miltenyi: Research Funding; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; NEKTAR: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Speakers Bureau; Omeros: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sobi: Honoraria, Speakers Bureau; Merck: Research Funding; Celularity: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal