Abstract

Introduction: Outcomes have improved for younger adults with BCP-ALL through better understanding of the biological mechanisms of BCP-ALL and development of intensive CT regimens (overall survival [OS], ≥60%). However, outcomes in patients (pts) >55 y remain poor (5 y OS, <10-40%) as toxicities limit use of intensive CT regimens. Novel treatment strategies incorporating immunotherapy to reduce CT-related morbidity and mortality may improve outcomes in this older pt population. Blinatumomab, a CD3/CD19-directed bispecific T-cell engager (BiTE®) molecule, directs T cells to lyse CD19+ B-lineage cells and has demonstrated efficacy in pediatric and adult pts with relapsed or refractory CD19+ BCP-ALL or minimal residual disease (MRD) ≥0.1%. We report results from the single-arm safety run-in for the randomized controlled phase 3 Golden Gate Study in older adults with newly diagnosed Ph-negative BCP-ALL (NCT04994717). The safety run-in evaluated blinatumomab alternating with low-intensity CT and a shorter blinatumomab dose step (4 days) than presently included in the blinatumomab label.
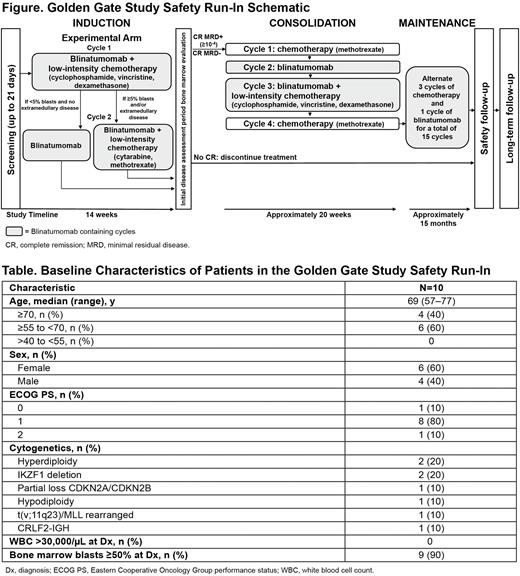
Methods: Pts aged ≥55 y (or 40 to <55 y with severe, pre-defined comorbidities) with newly diagnosed Ph-negative BCP-ALL, Eastern Cooperative Oncology Group performance status ≤2 and adequate organ function were included. Clearance of central nervous system (CNS) leukemia with intrathecal (IT) CT was required before enrollment. Pts in the safety run-in (Figure) were to receive 7 cycles (C) of blinatumomab (2 in induction, 2 in consolidation and 3 in maintenance) alternating with CT, including IT CT. Rituximab (8 doses) was given if CD20 expression was ≥20%. Permitted pre-phase CT consisted of cyclophosphamide, vincristine, dexamethasone and/or hydroxyurea; IT CT was requested per local standard of care. Primary objective of the safety run-in was to evaluate the safety and tolerability of blinatumomab alternating with low-intensity CT. Secondary objectives were to evaluate efficacy endpoints (complete remission [CR] within 14 weeks of starting induction C1; MRD response, defined as <10-4 by quantitative PCR [central lab] within 14 weeks of starting induction C1; relapse-free survival) and pharmacokinetics (PK) of blinatumomab.
Results: Ten pts enrolled in the safety run-in between November 2021 and 28 June 2022. Median age was 69 y (range, 57-77); 4 (40%) pts were ≥70 y (Table). Nine (90%) pts had bone marrow blasts ≥50% at diagnosis; 4 (40%) pts were CD20+ and received rituximab. All 10 pts completed induction C1. Seven pts remain on treatment (induction C2, n=5 [ongoing, n=2; completed, n=3]; consolidation C1 completed, n=1; consolidation C2 ongoing, n=1) and 3 pts discontinued treatment (2 for planned allogeneic stem cell transplant in post C2 induction CR and 1 due to isolated extramedullary relapse during induction C2). From the data cut off (28 June 2022), the most common all-grade treatment emergent adverse events (TEAEs) were cytokine release syndrome (CRS; n=5), decreased neutrophils (n=5) and decreased white blood cell count (WBC; n=5). All pts had grade ≥3 TEAEs; grade ≥3 TEAEs in ≥20% of pts were neutropenia (n=6), decreased WBC (n=5), anemia (n=3), thrombocytopenia (n=3) and bacteremia (n=2). All grade 4 TEAEs were hematologic. No deaths occurred. One pt had a serious adverse event (AE; grade ≥3, bilirubin increased). Grade ≥3 CNS TEAEs occurred in 1 pt (vasovagal and peripheral neuropathy unrelated to blinatumomab); no grade ≥3 CNS or CRS AEs related to blinatumomab occurred. Four pts had COVID-19 (grade 2, n=3; grade 3, n=1); blinatumomab was interrupted in 4/4 pts until COVID-19 negative and treatment then resumed without unusual AEs. After induction C1, all 10 pts achieved CR and 9/10 pts achieved a MRD response <10-4 per central lab. After induction C2, 5 pts had MRD results available; all 5 pts had a MRD response <10-4. Blinatumomab PK results will be presented.
Conclusions: Blinatumomab alternating with low-intensity CT had an acceptable safety profile and was well tolerated. No deaths occurred. The regimen was efficacious in this older adult population as all 10 pts achieved CR and 90% had a MRD response <10-4 after induction C1. These data support the planned regimen for the randomized controlled phase 3 study of blinatumomab alternating with low-intensity CT versus standard of care CT.
Disclosures
Jabbour:Genentech: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Amgen: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding. Aldoss:AbbVie: Consultancy, Research Funding; Agios: Consultancy, Honoraria; Amgen: Consultancy; Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Autolus Limited: Consultancy; Kite: Consultancy. Fleming:Gilead: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Honoraria, Speakers Bureau. Bajel:Abbvie: Honoraria; Amgen: Honoraria, Speakers Bureau; Astellas: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Takeda: Honoraria. Zugmaier:Amgen: Current Employment, Current equity holder in publicly-traded company; Micromet/Amgen: Patents & Royalties: Patents 20190300609 and 20130323247 licensed; Receives royalties of family members of international applications published as WO2010/052014; WO2010/052013; WO2011/051307; WO2012/055961; WO2012/062596; WO2014/122251; and WO2015/181683. Morris:Amgen: Current Employment. Chen:Amgen: Current Employment. Powar:Amgen: Current Employment. Wong:Amgen: Current Employment. Steffen:Jazz Pharmaceuticals: Other: Travel/Congress Participation Support; AbbVie: Other: Travel/Congress Participation Support. Kantarjian:Novartis: Honoraria, Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; Jazz Pharmaceuticals: Research Funding; AbbVie: Honoraria, Research Funding; NOVA Research: Honoraria; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Research Funding; Takeda: Honoraria. Goekbuget:AstraZeneca: Other: Invited talk for company-sponsored symposia (with honoraria); Cellestia: Other: Advisory board; Erytech: Other: Advisory board; AbbVie: Other: Research funding (institution); Pfizer: Consultancy, Other: Research funding (institution); invited speaker; advisory board; Amgen: Consultancy, Other: Research funding (institution); invited speaker; advisory board; Gilead/Kite: Other: Research funding (institution); invited talk; advisory board; Incyte: Other: Research funding (institution); advisory board; Jazz Pharmaceuticals: Other: Research funding (institution); advisory board; MorphoSys: Other: Advisory board; Novartis: Other: Research funding (institution); advisory board; Servier: Consultancy, Other: Research funding (institution); invited speaker; advisory board; Clinigen: Other: Advisory board.
OffLabel Disclosure:
Blinatumomab, a CD3/CD19-directed bispecific T-cell engager [BiTE(R)] molecule, directs T cells to lyse CD19+ B-lineage cells. The safety run-in evaluated blinatumomab alternating with low-intensity chemotherapy and a shorter blinatumomab dose step (4 days) than presently included in the blinatumomab label.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal