Abstract
Introduction: The most important treatment complication of hemophilia A is the development of neutralizing antibodies anti-factor VIII (inhibitors). Among the non-genetic risk factors for inhibitors development, the influence of the type of factor concentrate used in replacement therapy remains controversial.
Objective: The aim of this study was to systematically review the incidence of inhibitor development after treatment with recombinant factor FVIII (rFVIII) compared with plasma factor FVIII (pdFVIII) in previously untreated patients (PUPs) with severe hemophilia A.
Methods: A computer-assisted search of MEDLINE, Cochrane Library, EMBASE, Scopus, Google Scholar and Web of Science, without language restriction, between January 2010 and May 2022, was conducted using different combinations keywords. Other publications were selected from presentations from the largest conferences in the area, to identify relevant studies not detected in the electronic search. Additional studies were identified by contacting experienced researchers in the field and searching the reference list of primary studies. The articles were analyzed by two independent reviewers using pre-established eligibility criteria and in case of disagreement, a third independent reviewer was responsible for the final decision. To avoid double counting of the same cohort of patients, study periods were observed, and patients in duplicates were excluded. Data extraction form was made using the RedCap Platform. If some study data were missing or incomplete, an email was sent to the corresponding author to obtain the information. Quality assessment was performed on all included studies using The Newcastle-Ottawa Scale for Observational Cohort and Case Control Studies (NOS) and The Cochrane RoB 2.0 scale.
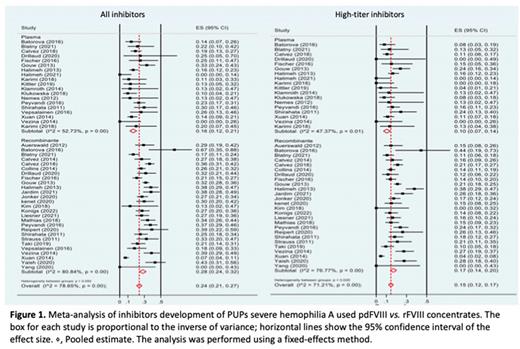
Results: Of the 974 potentially relevant publications initially selected, 34 were included in the final analysis, published between January 2010 to May 2022. Data from 4,244 patients enrolled in these studies were included in this analysis. Of these, 1,047 received pdFVIII, and 3,324 received rFVIII. The total number of patients who developed inhibitors was 1,136, of which 204 (19.5%) were treated with pdFVIII and 929 (28.8%) with rFVIII. High-titer inhibitors were found in 730/4,271 (17.1%) patients, 144/1,047 (13.8%) for pdFVIII and 585/3,224 (18.1%) for rFVIII. The pooled analysis of all studies showed inhibitor incidence rate of 17% for pdFVIII vs. 28% for rFVIII. Regarding high-titer inhibitors, the pooled incidence rate was 11% for pdFVIII studies compared to 17% for rFVIII (figure 1). Analyzing the different generations of rFVIII, it was observed the inhibitor incidence rate of 19% for first-generation rFVIII, 31% for the second-generation, 26% for third-generation and 27% for fourth-generation. Considering only the pooled incidence rate of high-titer inhibitors, it was observed 15%, 23%, 15% and 16 %, respectively. The inhibitor incidence rate in patients who received extended half-life (EHL) rFVIII was 28%, with 14% of high-titer inhibitor. Comparing pdFVIII concentrates to standard rFVIII, there was a statistically significant difference (p = 0.004). Regarding the two EHL-rFVIII concentrates with data available in the literature on PUPs (rFVIII-Fc [Elocta®] and N8-PEG [Esperoct®]) it was demonstrated also a statistical difference in inhibitor development compared to pdFVIII (p = 0.003). However, when compared inhibitor incidence rate of third-generation rFVIII and EHL-rFVIII, no statistical difference was observed. Nevertheless, the incidence rate of high-titer inhibitors among patients using pdFVIII and rFVIII, was statistically different only with second-generation rFVIII concentrates (p = 0.001).
Conclusions: In our systematic review and meta-analysis, which included 34 studies, an increase in the incidence of inhibitors was demonstrated in patients who used rFVIII when compared to pdFVIII. However, when analyzing only high-titer inhibitors, there was statistical difference only with second-generation rFVIII, which are not commonly used nowadays.
Disclosures
Prezotti:BioMarin: Consultancy, Research Funding; Takeda: Consultancy, Honoraria. Villaça:Takeda: Consultancy, Honoraria; Bayer: Consultancy; Novo Nordisk: Consultancy; Sanofi: Consultancy, Honoraria; BioMarin: Consultancy, Research Funding. Yamaguti Hayakawa:BioMarin: Consultancy, Honoraria; Roche: Honoraria. Ozelo:Pfizer: Consultancy, Honoraria, Research Funding; Novo Nordisk: Consultancy, Research Funding, Speakers Bureau; Bayer: Consultancy, Speakers Bureau; BioMarin: Consultancy, Research Funding, Speakers Bureau; Grifols: Research Funding; Roche: Research Funding; Sanofi: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal