Abstract
Background: Surgery and other invasive procedures have the potential for excessive, uncontrolled bleeding in patients with hemophilia A. Simoctocog alfa (Nuwiq®) has been shown to be highly efficacious at controlling bleeding during and after surgery in hemophilia A patients. Sufficient factor VIII (FVIII) must be available for the surgery and to maintain postoperative coverage. Emicizumab is indicated for the prophylaxis of bleeding in patients with hemophilia A, but additional hemostatic cover may be required to manage bleeding during surgery, particularly major surgeries. To date, there are limited data available on the use of FVIII to provide additional hemostatic cover for surgery in patients on emicizumab.
Aims: To evaluate the overall haemostatic efficacy of simoctocog alfa during major surgery in hemophilia A patients on regular emicizumab prophylaxis.
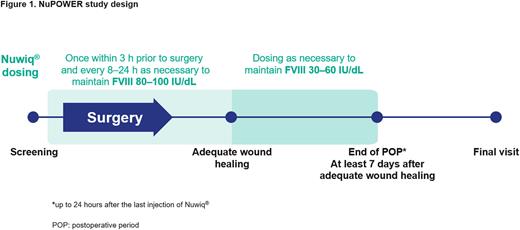
Methods: NuPOWER is a prospective, open-label, single-arm, multicenter study to be conducted at approximately 15 study centers worldwide. Previously treated male patients ≥12 years of age with severe hemophilia A (FVIII:C <1%) and no current or past FVIII inhibitors, who have been on regular emicizumab prophylaxis for at least 3 months and scheduled for major elective surgery are eligible for the study. Simoctocog alfa will be administered once within 3 hours prior to surgery and repeated as necessary every 8-24 hours to achieve a FVIII plasma level of 80-100 IU/dL until adequate wound healing has been achieved. Simoctocog alfa dosing will continue for at least another 7 days to maintain a FVIII plasma level of 30-60 IU/dL (Figure 1). The primary endpoint is the overall perioperative hemostatic efficacy ("success" or "failure") of simoctocog alfa. Hemostatic efficacy will be assessed at the end of surgery and at the end of the postoperative period (i.e. up to 24 hours after the last injection of simoctocog alfa), both using an objective 4-point ordinal scale, and adjudicated by the Independent Data Monitoring Committee using a pre-defined algorithm. Secondary endpoints include thrombin generation potential, and incidence of adverse events including thrombotic events and inhibitor formation. Target enrollment is 23 patients to document 26 major surgeries. Each subject can have multiple independent surgeries that will be counted as separate procedures. Study start is planned for Q4, 2022.
Conclusions: Results from the NuPOWER study will provide valuable data on the use of simoctocog alfa in patients on emicizumab prophylaxis who need to undergo major surgery. The data will help healthcare professionals to develop evidence-based guidelines for the management of bleeding in hemophilia A patients on emicizumab during surgery.
Disclosures
Werner:Octapharma USA: Current Employment. Knaub:Octapharma AG: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal