Abstract
Background: Allogeneic hematopoietic cell transplants (alloHCT) from HLA-matched sibling donors (MSD) or matched unrelated donors (MUD) may cure patients with acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), and myelodysplastic syndromes (MDS). Older patients with AML benefit from alloHCT, but donor selection may be complicated by the advanced age and comorbidities of HLA-matched siblings. Haploidentical (Haplo) HCT with post-transplant cyclophosphamide (PTCy) is an alternative approach to traditional HLA-matched alloHCT. HaploHCT is associated with reduced graft-versus-host disease (GVHD) risk but reduced overall survival (OS) in some series with older recipients. There are limited published data assessing outcomes using younger Haplo donors versus older MSD for older HCT recipients. The objective of this study is to characterize treatment outcomes and GVHD rates in older HCT recipients of younger HaploHCT compared to older MSD grafts.
Methods: This retrospective cohort analysis included patients aged 50 years and older with AML, ALL, or MDS who received reduced intensity conditioning (RIC) and either a young Haplo donor or older MSD graft from 2014 to 2020. Young donors included those <50 years old, and older donors were ≥50 years old. The primary outcome was OS one-year post-HCT. Secondary analyses included aGVHD and cGVHD outcomes, relapse-free survival (RFS), GVHD-RFS (GRFS), non-relapse mortality (NRM), infection incidence, time to neutrophil recovery, and relapse incidence. Kaplan-Meier method with log-rank test was used to assess OS, RFS, and GRFS. All data presented are organized as Haplo versus MSD.
Results: Twenty-nine patients received younger donor Haplo grafts and 47 patients received older MSD grafts. Median age for donors was 35.4 years vs 61.6 years in the Haplo and MSD cohort, respectively. The underlying hematologic disorder leading to alloHCT in the two cohorts were AML (83% vs 47%), ALL (3% vs 11%), and MDS (28% vs 43%) (p<0.001). Stratified cytogenetic risk categories for each diagnosis were not significantly different. RIC regimens included Fludarabine (Flu)/Melphalan or Flu/Busulfan for older MSD recipients, whereas the younger donor Haplo cohort received Flu/Cy/TBI (200cGy). PTCy/Tacrolimus (Tac) was the GVHD prophylaxis (ppx) regimen for all Haplo recipients, whereas 96% of MSD cohort got Tac/Methotrexate (MTX) and 4% of MSD cohort got Tac/MTX/MMF. Peripheral blood was the graft source in 62% of HaploHCT and 100% of MSD (p<0.001). Seventy-six percent of donors in the Haplo cohort were recipient offspring.
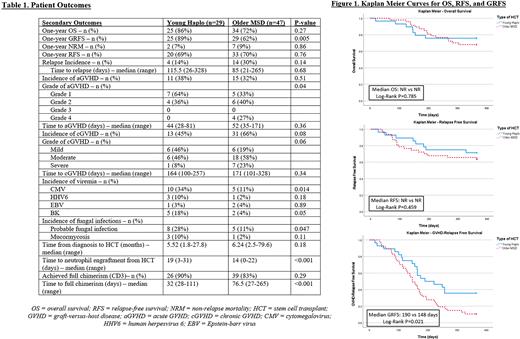
One-year OS was 86% vs 72% (p=0.27), RFS was 69% vs 70% (p=0.76), NRM was 7% vs 9% (p=0.86), and GRFS 89% vs 62% (p=0.005) [Table 1] for Haplo vs MSD cohorts, respectively. Median OS (HR 0.88, p=0.785) and median RFS (HR 1.38, p=0.459) were not reached in either group. Median GRFS was longer in the Haplo cohort (190 vs 148 days, HR 0.52, p=0.021) [Figure 1]. One-year incidence of relapse was not different between cohorts (p=0.14). Incidence of aGVHD (38% vs 32%, p=0.51) was similar in both cohorts, although maximum aGVHD grade was lower in the Haplo group (p=0.04). cGVHD incidence numerically favored HaploHCT (45% vs 66%, p=0.08) [Table 1]. Individuals receiving HaploHCT had a longer median time to neutrophil engraftment (19 vs 14 days, p<0.001) and a shorter median time to full CD3 chimerism (32 vs 76.5 days, p<0.001). They also had a higher incidence of CMV viremia (p=0.014), BK viremia (p=0.05), and fungal infections (p=0.047) [Table 1]. Incidence of HHV6 (p=0.18) and EBV viremia (p=0.89) were not different between cohorts.
Discussion: Our results demonstrate similar OS, RFS and incidence of aGVHD and cGVHD between younger donor HaploHCT and older MSD alloHCT for older patients. GRFS was significantly better in the Haplo cohort, which may have been primarily influenced by higher incidence of cGVHD and higher grades of aGVHD observed for recipients of older MSD grafts with Tac/MTX-based GVHD ppx. aGVHD and cGVHD rates may be reduced with the use of HaploHCT and incorporation of PTCy at the expense of more opportunistic infections. Our results suggest that young donor HaploHCT using PTCy may be a reasonable alternative for alloHCT and reduce risks for GVHD for older patients with leukemia even if a MSD is available. These data also suggest MSD alloHCT with PTCy should be explored.
Disclosures
Logan:Amgen: Research Funding; Amphivena: Research Funding; Astellas: Research Funding; Autolus: Research Funding; Jazz: Research Funding; Kadmon: Research Funding; Kite/Gilead: Research Funding; BMS: Consultancy; Pfizer: Consultancy; Pharmacyclics: Research Funding; Abbvie: Consultancy. Olin:Cellectis: Other: NA; Actinium: Consultancy; Astellas: Consultancy; Abbvie: Consultancy. Smith:Celgene: Research Funding; Erasca: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Revolution Medicines: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal