Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is curative in patients with sickle cell disease (SCD). HSCT using nonmyeloablative conditioning leads to a mixed chimerism that improves the SCD phenotype but may be complicated by the continued need for immunosuppression and graft loss. Granulocyte colony stimulating factor (G-CSF) may stimulate progenitor cells and is used post-HSCT to reduce the duration of neutropenia and the risk of neutropenic fever. G-CSF has been generally avoided in patients with SCD due to its risk of promoting vaso-occlusion (PMID 19513902).
Starting in 7/2016, our institution administered G-CSF after day +5 post-HSCT on the days when the ANC was < 500 x103/µL. In this study, we evaluated whether G-CSF is safe and can improve the duration of neutropenia, infection risk, and donor chimerisms in adults with SCD undergoing non-myeloablative HSCT. We included 34 adults with SCD that underwent allogeneic HSCT from an HLA-matched sibling donor in our institution and were conditioned with alemtuzumab and total body irradiation (TBI) (PMID 33534948) between 11/2011 and 8/2021. Medical records were individually reviewed for data extraction. Linear and categorical outcomes were compared by G-CSF use with the Kruskal-Wallis and chi-square test, respectively. We also compared the Kaplan-Meier curves for discontinuation of immunosuppression with the log-rank method. Median and interquartile range (IQR) are provided.
The median age of our cohort was 33 years (IQR, 26 - 45 years), 18 (53%) patients were female, and 30 (88%) had hemoglobin SS genotype. We did not observe statistically significant differences in baseline characteristics between the SCD patients that received G-CSF versus did not receive G-CSF for median age (37 years vs. 32 years), female gender (69% vs. 43%), hemoglobin SS genotype (85% vs. 90%) or median CD34+ dose (8.6 vs. 8.1 x 106/kg), respectively. In the 13 SCD patients that received G-CSF, the median day when it was initiated was day +10 (IQR, 7 - 17) and for a median of 3 days (IQR, 2 - 7 days). The maximum pain score during hospitalization was not significantly different in the SCD group that received G-CSF (median 8, IQR, 7 - 10) compared to those that did not receive G-CSF (median 10, IQR, 9 - 10). Median time to neutrophil engraftment (G-CSF: day 16 vs. no G-CSF: day 22) and duration of neutropenia (G-CSF: 7 days vs. no G-CSF: 9 days) were relatively similar between the 2 groups, while the incidence of neutropenic fevers trended lower with G-CSF treatment (8% vs. 29%, respectively, P=0.1).
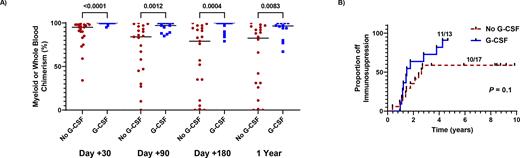
Whole blood or myeloid chimerism values were significantly higher in the SCD patients that received G-CSF at day +30, +90, +180, and 1 year post-HSCT (Figure 1A). Secondary graft loss was not observed after we implemented G-CSF while 4 out of 21 (19%) of patients experienced secondary graft failure who did not receive post-HSCT G-CSF. Furthermore, in those patients with stable engraftment, we observed a trend for more patients who received G-CSF being successfully tapered off immunosuppression (11/13, 85%) versus those that did not receive G-CSF (10/17, 59%) (Figure 1B).
In conclusion, administration of G-CSF to patients with SCD undergoing HSCT conditioned with alemtuzumab/TBI was associated with significantly higher whole blood or myeloid donor chimerism values. This benefit was achieved without a significant increase in pain scores during the hospitalization for HSCT and was associated with trends for a greater proportion of patients discontinuing immunosuppression and less secondary graft loss. Future studies investigating the effects of G-CSF on donor myeloid progenitor cells and on T-cell clearance may guide strategies to improve outcomes for curative therapies in adults with SCD.
Disclosures
Patel:Exelixis: Current Employment. Gordeuk:Forma: Consultancy; GSK: Consultancy; GBT: Consultancy, Research Funding; CSL Behring: Consultancy, Research Funding. Saraf:FORMA Therapeutics: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding, Speakers Bureau; Agios: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; ORIC: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal