Abstract
Cytomegalovirus (CMV) infection remains a common complication after allogeneic hematopoietic- cell transplantation (HCT) with a significant increase in morbidity and mortality among patients who acquire such infection. Pre-emptive prophylaxis in which antiviral agents are started based on detection of CMV in the blood to prevent clinical disease has been the standard practice for many years. This approach was limited by the toxicity of available anti-CMV medications and increased complications among patients who activate CMV(1, 2). Letermovir, an antiviral agent that inhibits replication by binding to components of the terminase complex (UL51, UL56, or both) was approved in 2017 for prevention of CMV infection and disease among HCT recipients(3). Letermovir has been shown to delay polyfunctional CMV-specific cellular immune reconstitution as well as cause a decrease in absolute polyfunctional CD8+ T cell responses(4). The impact of such delayed immune reconstitution on infections other than CMV has not been widely described especially in the setting of post-transplant cyclophosphamide as GVHD prophylaxis.
To study the impact of Letermovir on non-CMV infections during the first 6 months post HCT and to assess its effect on other transplant endpoints including OS, DFS, NRM, relapse, acute and chronic GVHD, we analyzed 409 consecutive patients who received their HCT at our center between 2016 and June of 2021. Letermovir prophylaxis was started at our center in January 2018 and is given to patients receiving MUD or haploidentical transplant and are either CMV+ or donor is CMV+. The final dataset included 321 subjects with the following characteristics: Median age 54 years (19-80); disease AML/ALL/MDS 81%, NHL/HD/Other 19%; donor type haplo (69%), MUD 31%; Cell Source PBSC 88%; Myeloablative conditioning 38% ; Donor/Recipient +/+ 50%, +/- 12%, -/+ 38% . The median follow up for survivors was 31.2 (11.5-52.6) months for letermovir group versus 66.2 (53.1-76.9) months for no letermovir group. Patients in the letermovir group were less likely to have received a reduced intensity regimen (22% vs 33%, p=0.05) and to have received a marrow graft (8% vs 16%, p=0.02).
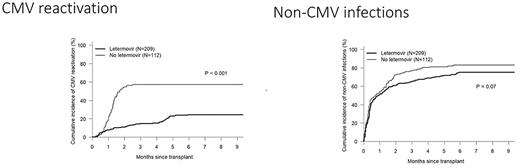
The survival endpoints at 3 years were OS (72.7% vs 68.8% p=0.5), DFS (64.4% vs 58.0% p=0.27), relapse (21.2% vs 28.6% p=0.08) and non-relapse mortality NRM (14.5% vs 13.5% p=0.5) for letermovir compared to no letermovir . The cumulative incidences for grade II-IV acute and moderate-severe chronic GVHD were 50.7% vs 40.2%, p=0.08 and 24.3% vs 24.1%, p=0.85 for Letermovir and no letermovir respectively The cumulative incidence of CMV reactivation was significantly lower for letermovir 24.4% compared to 57.1% for letermovir (p<0.001) figure 1, whereas non-CMV infections were similar between both groups with a trend towards lower infections for letermovir recipients 75.2% vs 83% p=0.07, figure 1. When assessing the difference in non-CMV infections per infection, there was no difference in the mean number of infections at 6 months post-HCT where patients in both groups had a mean of 2.5 infections. When looking at different types of infections between Letermovir and no letermovir groups, there was no difference in mean number of bacterial infections (1.58 vs 1.43), viral infections (0.90 vs 0.95) and fungal infections (0.16 vs 0.22), P =NS for all.
Our data shows that in a large cohort of allogeneic HCT recipients, mostly haploidentical with Post-transplant cyclophosphamide, letermovir significantly decreases CMV reactivation without changing the pattern or incidence of non-CMV infections.
1. Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427-38.
2. Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113(23):5711-9.
3. Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. The New England journal of medicine. 2017;377(25):2433-44.
4. Zamora D, Duke ER, Xie H, Edmison BC, Akoto B, Kiener R, et al. Cytomegalovirus-specific T-cell reconstitution following letermovir prophylaxis after hematopoietic cell transplantation. Blood. 2021;138(1):34-43.
Disclosures
Solh:ADC Therapeutics: Research Funding; Partner Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal