Abstract
Objectives: Using BT with commercially available CAR T-cell therapy for treatment of r/r LBCL is at the discretion of treating physicians. With emerging real-world data on CAR T-cell therapy, we conducted an SLR to understand the patterns of BT use in real-world settings and to investigate its association with effectiveness and safety outcomes.
Methods: We systematically searched EMBASE, MEDLINE, and 15 conferences through April 1, 2022 for real-world observational studies describing patterns of BT use among adult patients receiving CAR T-cell therapy for r/r LBCL. In addition to data on the use of BT, the estimated effects of BT on clinical outcomes were also extracted. Weighted means were calculated using both fixed- and random-effects meta-analyses for use and type of BT, study location, and CAR T-cell product. The associations between use of BT and post-infusion overall and complete response rate (ORR and CR rate), progression-free survival (PFS), overall survival (OS), cytokine release syndrome (CRS), and neurotoxicity (NT) were assessed by meta-analyses of the reported effects of BT, with adjusted and unadjusted treatment effects analyzed separately.
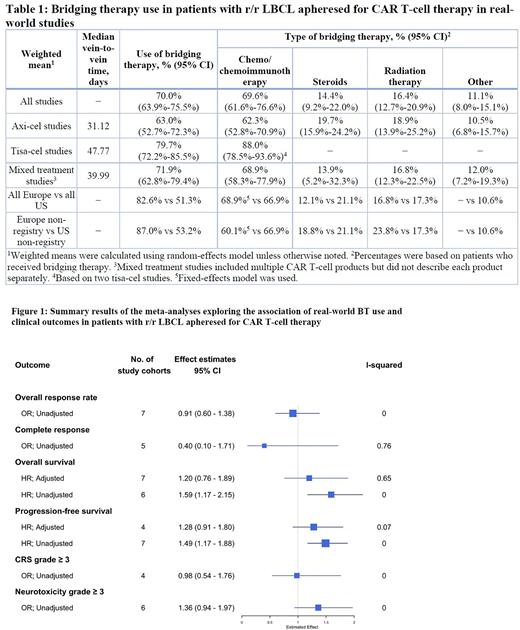
Results: The search identified 4,242 citations; 65 publications on 42 studies reporting on real-world use of BT were included in the evidence base, of which 32 publications on 25 studies reported on the associations between BT and clinical outcomes. No real-world study on lisocabtagene maraleucel was found at the time of the search. At baseline (apheresis or infusion), patients receiving axicabtagene ciloleucel (axi-cel) were younger compared with patients receiving tisagenlecleucel (tisa-cel) (weighted average of median age 59.5 years for axi-cel vs 62.6 years for tisa-cel), while more likely to have an International Prognostic Index (IPI) of ≥ 3 (51% for axi-cel vs 41% for tisa-cel), and more likely to have bulky disease (24% for axi-cel vs 16% for tisa-cel); sex, disease stage, double/triple hit, number of prior lines of therapy, prior history of autologous stem cell transplant, and refractory status were comparable between the two products. A shorter median time from leukapheresis to infusion (vein-to-vein time) for axi-cel was observed in both US- (weighted average 27.7 d for axi-cel vs 44.0 d for tisa-cel) and European-based studies (40.0 d for axi-cel vs 49.3 d for tisa-cel).
On weighted average, 63% (95% confidence interval [CI] 53%-72%) of axi-cel recipients received BT compared with 80% (95% CI 72%-86%) of tisa-cel recipients (Table 1). Overall, use of BT was also more common in Europe (83%) than in the US (51%). Among patients who received BT, the most common type was chemo/chemoimmunotherapy (70% overall; 62% for axi-cel; 88% for tisa-cel) followed by steroids in the US (21% vs 12% in Europe) or radiation therapy in Europe (17% vs 17% in the US). Response to BT was seldomly reported and heterogeneous.
Meta-analyses based on 7 available real-world cohorts (6 studies) that reported adjusted results on OS did not provide sufficient evidence to indicate an association between use of BT and OS (hazard ratio [HR] 1.20; 95% CI 0.76-1.89; Figure 1). Use of BT was also not associated with PFS based on adjusted results of 4 available cohorts (HR 1.28; 95% CI 0.91-1.80). Limited to unadjusted results only, no significant associations were found between use of BT and ORR (odds ratio [OR] 0.91; 95% CI 0.60-1.38), CR rate (OR 0.40; 95% CI 0.10-1.71), CRS of grade ≥ 3 (OR 0.98; 95% CI 0.54-1.76) or NE of grade ≥ 3 (OR 1.36; 95% CI 0.94-1.97).
Conclusions: These findings provide a comprehensive picture of patterns of BT use in real-world settings, notably its more frequent use in Europe and among patients receiving tisa-cel. Despite the more frequent use of BT with tisa-cel, patients receiving tisa-cel did not appear to have more severe disease at baseline, suggesting that the shorter vein-to-vein time for axi-cel may have contributed to the reduced use of BT among its recipients. Given the use of BT could be heavily confounded by unknown factors that affect physicians’ decisions, the lack of adjusted analyses limit the conclusions that can be drawn on its association with clinical outcomes. Prospective studies evaluating the value of integrating BT with CAR T-cell therapy are warranted.
JM and ZHH contributed equally.
Disclosures
Muñoz:Curio: Honoraria; Kyowa Kirin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; OncView: Honoraria; Physicians’ Education Resource: Honoraria; Seagen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Targeted Oncology: Honoraria; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Debiopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Fosun Kite: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genmab: Consultancy, Membership on an entity's Board of Directors or advisory committees; Innovent: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MorphoSys/Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics/AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acrotech/Aurobindo: Speakers Bureau; AstraZeneca: Speakers Bureau; Celgene: Research Funding, Speakers Bureau; Genentech/Roche: Speakers Bureau; Kyowa: Speakers Bureau; Pharmacyclics/Janssen: Speakers Bureau; Verastem: Speakers Bureau; Genentech: Research Funding; Gilead/Kite Pharma: Research Funding; Incyte: Research Funding; Merck: Research Funding; Millennium: Research Funding; Pharmacyclics: Research Funding; Portola: Research Funding. Hu:Kite, a Gilead Company: Current Employment. Kanters:RainCity Analytics: Research Funding. Limbrick-Oldfield:RainCity Analytics: Current Employment. Miao:Gilead Sciences: Current holder of stock options in a privately-held company; Kite, a Gilead Company: Current Employment. Spooner:Kite, a Gilead Company: Current Employment; Gilead Sciences: Current holder of stock options in a privately-held company; Delta Hat Limited: Other: Support with conception, analysis and manuscript writing. Commissioned by Kite, a Gilead company. Xu:Kite, a Gilead Company: Current Employment. Sanderson:Kite/Novartis: Honoraria, Other: Travel support, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal