Abstract
Introduction: With the development of various BCR-ABL1 tyrosine kinase inhibitors (TKIs), chronic myeloid leukemia (CML) has become a manageable hematologic malignancy and current therapeutic goal is treatment free remission (TFR). To achieve a successful TFR in more patients, a precise assessment of minimal residual disease (MRD) has become one of the prerequisite requirements. Since undetectable BCR-ABL1 by conventional TaqmanTM-based quantitative polymerase chain reaction (qPCR) is not an indicator for complete eradication of CML clone, more precise methods should be developed. Currently, to overcome some limitations of conventional qPCR, such as rigorous standardization process and various sensitivity between laboratories, digital quantitative-PCR (dqPCR) methods such as nanofluidic or droplet dqPCR have been developed for accurate and precise detection of various genes and its use in the routine clinical practice is gradually expanding. However, current dqPCR methods may have limitations due to partition-based amplification and end-point interpretation. Therefore the establishment of clinical guideline is crucial for wide application on TFR in CML. Therefore, we have developed a novel technology, a chip-based real-time dqPCR platform (LOAA; Lab On An Array; so called Dr. PCR™) to achieve more stable, accurate and precise monitoring of BCR-ABL1 in a timely manner.
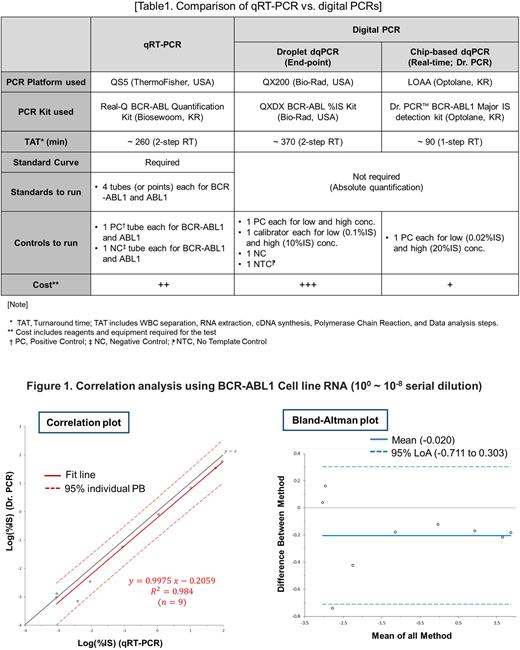
Methods: To assess the equivalence between Dr. PCR and conventional qPCR, we evaluate the efficacy of BCR-ABL1 detection using cell lines and clinical specimens. For linearity assessment, we used BCR-ABL1 positive (e14a2) K562 cell line diluted to 9 points by 10-fold serial dilutions from 100 to 10-8 with BCR-ABL1 negative HL60 cells. With the results from cell lines, we also evaluated linearities for both e13a2 and e14a2 major BCR-ABL transcripts using 7 clinical specimens obtained from Leukemia Omics Research Institute of Eulji University.
In detail, conventional qPCR was performed using Transcriptor First Strand cDNA Synthesis Kit (Roche, USA) for cDNA synthesis and Real-Q BCR-ABL Quantification Kit (Biosewoom, South Korea) for qPCR. Total 800ng of RNA per test was used for qPCR (QS5, Thermo Fisher, USA) with 8 replicates and duplicate for cell line and clinical specimens, respectively. For Dr. PCR (1-step reverse transcriptase PCR), 400ng (for cell lines) or 500ng (for clinical specimens) of RNA per test was applied to disposable cartridges of Dr. PCR™ BCR-ABL1 Major IS detection kit (Optolane Technologies, South Korea) with LOAA platform (Optolane Technologies, Korea). The copies of BCR-ABL1 and ABL1 were analyzed by LOAA analyzer S/W (Optolane Technologies, South Korea).
Based on the results, correlation analysis between qPCR and Dr PCR was performed using correlation graphs and Blend-Altman plots.
Results: Correlation analysis for cell line dilution assay showed that measured values from both qPCR and Dr. PCR were closely correlated with the R2 of 0.984. LOA95 (limit of agreement at 95% confidence interval) analyzed by Blend-Altman plot was -0.711 ~ 0.303 (mean = 0.02), indicating the two methods are closely correlated (Figure 1). The linearity assessment for e13a2 and e14a2 sub-genotypes using primary cells from 7 clinical specimens of CML patients again showed a close correlation with the R2 of 0.984 and 0.985, respectively. LOA95 values were -0.516 to 0.384 (mean = -0.066) and -0.407 to 0.361 (mean = -0.023), respectively. Interestingly, Dr PCR efficiently detected clinical specimens with low copy number of BCR-ABL1 compared to conventional qPCR, which is beneficial for the determination of MDR levels in patients especially those who are required to monitoring after cessation of TKIs. Comparison between conventional qPCR, droplet dqPCR and Dr PCR is listed (Table 1). Results with more specimens from CML patients and additional information will be updated at ASH presentation.
Conclusion: Taken together, we believe our novel chip-based real-time dqPCR (Dr. PCR™) may minimize false positive results and maximize the analytical sensitivity of detecting BCR-ABL1 fusion transcripts and thus, would be useful for the selection of therapeutic option in routine clinical practice and TFR. We will include the result for further intensive study using more clinical specimens including samples of low copy number of BCR-ABL1.
Disclosures
Kim:Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Il-Yang: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Otsuka: Consultancy, Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal