Abstract
Background: Patients (pts) with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) undergoing second-line chemoimmunotherapy (2L CIT) often have a poor prognosis with a minority achieving curative outcomes. Achievement of CR with 2L CIT is associated with favorable long-term outcomes in patients consolidated with autologous stem cell transplant (ASCT). Unfortunately, only 25-35% of patients achieve complete response (CR/CR unconfirmed) with RICE CIT (Gisselbrecht 2010). Addition of novel targeted agents such as Bruton Tyrosine Kinase inhibitors (BTKi) to 2L CIT may offer improved treatment responses given the importance of B-cell receptor (BCR) signaling in DLBCL. Here we report the feasibility and efficacy of combining acalabrutinib (acala) 100mg BID with RICE (A-RICE) chemotherapy in ASCT eligible pts with R/R DLBCL.
Study Design and Methods: In a single-center, open-label, phase 2 trial (NCT03736616) we evaluate the feasibility, efficacy, and tolerability of combining acala with RICE as 2L CIT in R/R DLBCL pts. There are two study cohorts. Cohort A is open to R/R DLBCL pts who are eligible for 2L CIT followed by ASCT consolidation. Cohort B is open to R/R DLBCL pts considered medically ineligible for ASCT. The primary objective for cohort A is to estimate the confirmed CR rate (RECIL 2017 criteria) of A-RICE prior to HCT. Primary endpoint for cohort A is met if >10 of maximum 24 enrolled patients achieve CR, which allows rejection of the null hypothesis that confirmed CR rate is ≤ 25% if true CR rate is 50% (one-sided α =0.05, power = 85%). The primary endpoint for cohort B is the estimate of one-year progression-free survival in patients undergoing 2L CIT followed by maintenance acala for up to 12 months. Secondary endpoints include overall response rate (ORR), incidence of Grade 3/4 adverse events (AEs), and incidence of serious AEs in both cohorts.
Pts in cohort A received 2 cycles of A-RICE in a 21 day cycle. After 2 cycles A-RICE, response assessment via PET-CT (PET2) was completed with responding patients receiving a 3rd cycle of A-RICE followed by stem cell mobilization and collection. PET-CT was performed 14-21 days after day 1 of cycle 3 (PET3) to assess response to 2L CIT. Those patients with CR or partial response (PR) after PET3 proceeded to BEAM conditioned ASCT within 28-42 days of PET3. After hematopoietic recovery, patients may continue acalabrutinib 100mg BID as maintenance therapy for 12 months post ASCT. Minimal residual disease (MRD) is assessed using ctDNA (clonoseq) at time points pre-ASCT, post-ASCT, and during maintenance A.
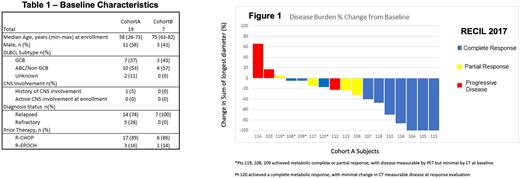
Results: Primary endpoint for Cohort A has been met and is reported here, while Cohort B has not yet met pre-specified enrollment or follow up maturity for efficacy analyses. Safety for both cohorts to date is reported. Twenty-six pts have been enrolled (19 cohort A, 7 cohort B). In Cohort A, 5 pts had refractory DLBCL, 7 pts were GCB, 10 non-GCB. Median age of Cohort A was 58, and median of Cohort B was 75 (Table 1). 19 Cohort A pts received at least 1 cycle of A-RICE, with 16 pts completing 3 cycles. One patient (4%) stopped A-RICE due to AE, 3 patients (13%) discontinued due to progressive disease (PD). All 19 pts in cohort A were considered response evaluable following initiation A-RICE: ORR was 74% (14 pts), with 53% CR (10 pts), 21% PR (4 pts). Thirteen pts (68%) underwent planned consolidative ASCT. See Figure 1 for response data.
Safety data for all 26 cohort A and B patients who received at least 1 cycle A-RICE was assessed for the first three cycles of A-RICE. The most common treatment-related AEs were thrombocytopenia (All 50%, Gr 3/4 46%) and neutropenia (Gr 3/4 30%). SAEs were reported in 5 pts with 1 therapy related SAE of neutropenic fever, and 4 treatment unrelated SAEs.
Of the 19 efficacy evaluable pts in cohort A, 10 pts interrupted 1 or more doses of acalabrutinib during a A-RICE cycle either due to patient error or protocol specified dose hold related to AE.
Conclusions: A-RICE in ASCT eligible pts w/ R/R DLBCL demonstrated CR in 53% of response evaluable patients. Further, we observed a high ORR (74%) and high proportion of pts completing planned ASCT (68%). AEs with A-RICE were consistent with those expected for CIT. A-RICE warrants further investigation in pts w/ R/R DLBCL eligible for 2L CIT with intention to undergo ASCT. Further analyses of cohort A, including ctDNA based MRD dynamics, PFS, and OS are ongoing and will be updated at meeting.
Disclosures
Glennie:Pharmacyclics/Janssen: Speakers Bureau. Patel:Loxo Oncology: Consultancy, Research Funding; Kite pharma: Consultancy, Research Funding, Speakers Bureau; Epizyme: Consultancy, Research Funding; Abbvie: Consultancy; Xencor: Consultancy, Research Funding; Morphosys: Consultancy; MEI Pharma: Consultancy, Research Funding; CRISPR Therapeutics: Research Funding; Celgene: Consultancy, Research Funding, Speakers Bureau; Caribou Biosciences: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; BeiGene: Consultancy; Aptevo Therapeutics: Research Funding; ADC Therapeutics: Consultancy; Adaptive Biotechnologies: Research Funding; Velos Bio: Research Funding; Trillium Therapuetics/Pfizer: Consultancy, Research Funding; TG Therapeutics: Consultancy, Speakers Bureau; Nurix: Research Funding; Pharmacyclics/Janssen: Consultancy, Research Funding, Speakers Bureau; Sunesis Pharmaceuticals: Research Funding; Fate Therapeutics: Research Funding; Genetech/Roche: Consultancy, Research Funding, Speakers Bureau; Curis, Inc: Research Funding.
OffLabel Disclosure:
Acalabrutinib is not FDA approved for use in DLBCL and is an investigational agent in the study presented. We will disclose this during oral or poster presentation.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal