Abstract
iASPP is a member of the ASPP family of proteins and an evolutionarily conserved inhibitor of p53. iASPP is further an independent oncogene and an anti-apoptotic mediator, directly interacting with apoptosis-regulating proteins such as p53, Bcl-2 or NF-κB. Importantly, overexpression of iASPP has been described in several cancers and is associated with poor outcome, eg. in glioma or breast cancer entities. We have evidence that high expression of iASPP mediates a more aggressive biology and is involved in resistance to therapy in acute myeloid leukemia (AML).
To define the role of iASPP in acute myeloid leukemia (AML) we screened 66 unselected patients with newly diagnosed AML. iASPP expression patterns were evaluated by flow cytometry and qRT-PCR on protein and mRNA levels, respectively. A pool of mononuclear cells derived from the bone marrow of consented, healthy donors, served as a control for basal iASPP levels. To demonstrate direct functional consequences of iASPP in leukemogenesis and therapy response, iASPP-interferenced leukemia cell models were established (silencing efficacy of 70%, p=0.01, in MOLM14 and 65%, p=0.05, in Jurkat cells) using a lentiviral transduction approach. To assess iASPP expression patterns in dependence of clinical survival parameters, a well-defined cohort of 274 AML patient samples treated in the HOVON102 trial, a prospective randomized protocol investigating the therapeutic benefit of clofarabine in addition to standard induction chemotherapy, was used. Additionally, an independent large patient cohort of 1105 samples deriving from four clinical trial transcriptomic datasets (GSE1159, 12417, 6891, 8970) with follow-up data (avg. 41.2 mo) served as a validation cohort.
We here demonstrate that iASPP is frequently overexpressed in AML. Importantly, iASPP expression is significantly higher in AML patients compared to mononuclear cells of healthy bone marrow donors (p≤0.0001). No differences were found between blasts derived from bone marrow or peripheral blood.
In vitro modeling using iASPP silenced cell strains, demonstrates a decrease in cellular proliferation rates (cell doubling time in MOLM14_shiASPP 16.5 hrs versus 13 hrs in the empty vector (EV) controls, p=0.028 and Jurkat_shiASPP 23.5 hrs versus 13.3 hrs in the respective EV strains, p=0,0003). This observation argues for a direct role of iASPP in leukemogenesis and aggressivenesss of leukemia biology.
In addition, we show, that iASPP-interference results in enhanced induction of apoptosis compared to the EV cell strains, arguing for a role of iASPP in the context of resistance towards chemotherapy. Induction of apoptosis was restored in iASPP-silenced cells when treated with daunorubicin with an IC50 of 39.0 nM (MOLM14_shiASPP) compared to 92.6 nM (MOLM14_EV), p=0.0001. Similar results were observed for Jurkat cells, where treatment with daunorubicin or cytarabine resulted in an IC50 for Jurkat_shiASPP of 32.2 nM and 224 nM compared to 58.6 nM and 348 nM for the EV controls. Targeted inhibition of FLT3 ITD-mutant MOLM14 cells using sunitinib resulted in a favorable IC50 of 51.7 nM (MOLM14_shiASPP) vs. 82.8 nM (MOLM14_EV).
In line, analysis of the HOVON102 trial cohort of pts undergoing intensive chemotherapy, revealed that pts expressing low iASPP had a more favorable outcome with respect to overall survival (OS) (55.4 vs. 37.8 months, p=0,0004) as well as event free survival (EFS) (58.1 vs. 30.9 months, p=0,0004) as determined by statistical Wilcoxon test.
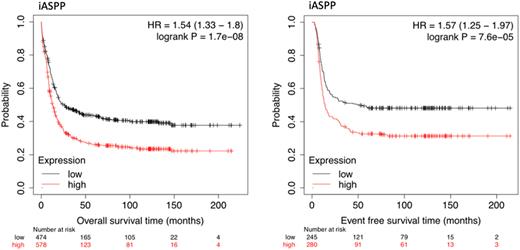
These observations were confirmed in the validation cohort (figure 1), where iASPPlow expressors had a significantly better median OS and EFS of 26 mo and 50.8 mo vs. 12.9 mo and 13.9 mo in the iASPPhigh cohort. The adverse influence of higher iASPP expression regarding clinical outcome was also maintained within molecular subentities - defining high risk groups not reflected by the ELN score (e.g. median OS in NPM1-mut/iASPPlow 43.6 mo vs. 19.2 mo in NPM1-mut/iASPPhigh).
To summarize, our data demonstrates that iASPP contributes to leukemogenesis and aggressiveness of the disease. Importantly, iASPP contributes to therapy resistance - and may serve as an additional prognostic marker for risk stratification in AML. Further prospective evaluation is warranted.
Disclosures
Schittenhelm:University of Tuebingen: Patents & Royalties: patent with respect to a new identified splicing variant - not related to this abstract; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees. Kampa-Schittenhelm:University of Tuebingen: Patents & Royalties: patent for alternative splice variant, unrelated to this topic.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal