Abstract

Introduction: Dyskeratosis congenita (DC) and related telomere biology disorders (TBD) result from germline variants in genes required for telomere maintenance. DC is traditionally characterized by a triad of nail dysplasia, oral leukoplakia, and reticular skin pigmentation. Significant complications include bone marrow failure (BMF), pulmonary fibrosis, liver disease, and hematologic and solid organ malignancies. Previous reports described avascular necrosis (AVN) of the femoral head or humerus in DC/TBD. However, these findings were limited to a few individual case reports. Herein, we present a study of AVN and minimal trauma fractures (MTF) in a large cohort of patients with DC/TBD.
Methods: We reviewed medical records of all patients with a confirmed diagnosis of DC/TBD enrolled in the IRB-approved Inherited Bone Marrow Failure Syndromes study (NCT00027274) between 2002 - 2019 for the history of AVN and/or MTF. Data were compiled on DC/TBD characteristics, treatments, as well as body mass index (BMI), lipid profile, bone mineral density (BMD), and 25-hydroxyvitamin D (25[OH]D) levels. We used stratified random sampling to match DC/TBD patients with AVN and/or MTF by age, gender, inheritance pattern and genotype with a control group of DC/TBD patients with no AVN/MTF. Distributional variance was assessed for all t-tests and two-sided p values <0.05 were considered significant. Statistical analysis was carried out using RStudio (Ver 1.4.1106).
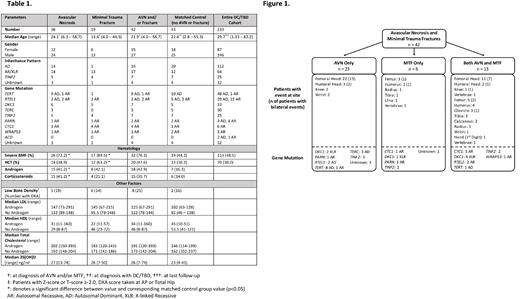
Results: Forty-two of 233 patients (18%) with DC/TBDs experienced at least one AVN and/or MTF at a median age of 21.9 years (range 4 to 58.7); 15/42 were females (Table 1). Twenty-three patients had AVN alone, 6 had MTF alone, and 13 developed both AVN and MTF (Figure 1). The median age at first AVN was 24.1 years (range 6.3 - 58.7) and at first MTF 13.6 years (range 4 - 44.3). AVN was more common in patients with autosomal recessive/X-linked recessive (AR/XLR)/TINF2 DC (n=20, 21.3%) and developed at a younger age (median 18.8 years, range 6.3 - 47.8) than in patients with autosomal dominant (AD) disease (12.5%; median age 29.9 years, range 11.1 - 58.7); p=0.013. MTF occurred almost exclusively in patients with AR/XLR/TINF2 DC (n=17, 19%) compared with AD DC/TBD (n=1, .9%); p<.0001.
There were 68 AVN events in 36 patients (median 2 events/patient, range 1 - 5). The most frequent sites were femoral heads (33/36) followed by humeral heads (5/36) and knees (3/36); 22/33 had bilateral femoral head AVN (Figure 1). Twenty-two of 26 patients with severe BMF received androgen/steroids within 5 years of AVN and 8 received hematopoietic cell transplantation (HCT). Compared with the matched control group, significantly more DC patients with AVN had severe BMF and received androgen/corticosteroids (p<0.02). Importantly, 12/36 DC/TBD patients (33%) developed AVN without prior HCT, corticosteroid, or androgen treatment. 24/36 patients received treatment for AVN, including total hip arthroplasty (n=15) and core decompression (n=5) or other medical intervention (n=4). Median time from diagnosis with AVN to treatment was 0.81 years (range 0.03 - 2.7).
We identified 38 MTF events in 19 DC patients; the most frequent fracture site was the femoral diaphysis (11 events in 8 patients) (Figure 1). Fractures occurred spontaneously or during routine activities such as entering a vehicle, running in the playground, or low-impact dancing as per patient reports. Seventeen patients (89%) with MTF had severe BMF, 12 (63%) had received HCT (7 had taken androgens/steroids). Compared with matched controls, significantly more DC patients with MTF had received HCT (p=0.02).
Other factors such as BMD, elevated cholesterol, overweight/obesity, and 25[OH]D levels were not significantly associated with AVN or MTF in our cohort.
Conclusions: AVN and MTF are highly prevalent in patients with DC/TBDs. AVN developed across the entire DC/TBD spectrum but was significantly associated with severe BMF and AR/XLR/TINF2 DC. MTFs occurred almost exclusively in young patients with severe disease indicated by AR/XLR/TINF2 mode of inheritance. Improved screening and effective clinical management of symptoms for patients at higher risk of developing AVN/MTF is needed. Furthermore, these findings signal the need for future research exploring the mechanism underlying AVN and MTF in patients with DC/TBDs.
Disclosures
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal