Abstract
Introduction Patients with end-stage liver disease have a very low amount of pro- and anticoagulants in their blood, and therefore they may develop both bleeding and thrombotic complications during liver transplant surgery. These patients are in need for optimized intraoperative transfusion to keep their coagulation system in balance, which is currently managed by whole-blood viscoelastic assays such as TEG. However, TEG is not standardized for coagulation analysis because of its high variability and requires a relatively large blood volume per test. Here, we demonstrate that our noncontact drop-of-blood technique, referred to as "integrated quasi-static acoustic tweezing thromboelastometry" (i-QATTTM), can accurately assess changes in the coagulation system during liver transplantation.
Method We have conducted side-by-side coagulation measurements by TEG and i-QATTTM. Whole blood samples (3-5mL each) were collected from liver transplant patients at the following time points: 1) immediately after induction of anesthesia (baseline), 2) after arterial reperfusion (artery), and 3) during biliary reconstruction (final). The samples were first used for TEG analysis, and their leftovers (waste blood) were analyzed by i-QATTTM.
i-QATTTM has two graphical outputs: mechanical tweezograph showing sample firmness change with time (similar to TEG tracing, Fig 1A) and photo-optical tweezograph showing sample turbidity change with time. The following parameters extracted from these curves were analyzed: clot initiation time (CIT), time to firm clot formation (TFCF), maximum clot firmness (MCF), and maximum fibrin level (MFL). Correlation analysis was performed between these parameters and the corresponding TEG output. The study was approved by Tulane University and Ochsner Medical Center IRBs.
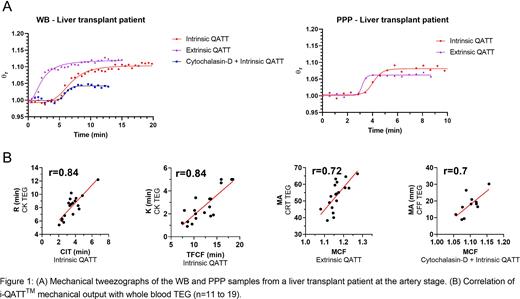
Results Figure 1A (left) shows the mechanical tweezographs of the WB sample from a liver transplant patient at the artery stage, exposed to intrinsic and extrinsic pathway activators, and a combination of Cytochalasin-D and intrinsic pathway activator. These tests correspond to CK, CRT, and CFF assays in TEG. Fig 1A (right) shows the mechanical tweezographs of the PPP sample from the same patient, triggered by intrinsic and extrinsic pathway activators. Noted that the WB treated with Cytochalasin-D has the MCF value reduced dramatically from that of untreated blood and nearly equal to the MCF level of the PPP.
In Fig. 1B, there were a strong linear correlation (Pearson correlation coefficient r > 0.72) between CIT and R-time of TEG (CK assay) and between TFCF and K-time of TEG (CK assay) for WB samples triggered via intrinsic pathway. Strong correlation also exists between MCF and MA values of TEG (CRT assay) for WB triggered via extrinsic pathway. MCF and MFL of PPP triggered by either pathway were strongly correlated (r > 0.74) with MA values of TEG (CFF assay). Strong correlation was also observed (r=0.7) between MCF and MA of TEG (CFF assay) for Cytochalasin-D treated WB triggered via intrinsic pathway.
For both activation pathways, CIT at the artery stage (3.7±0.6 min and 1.5±0.3 min, respectively) is slightly lower than at the baseline (4.0±0.5 min and 1.7±0.4 min) and final stages (3.9±0.4 min and 1.9±0.3 min) while TFCF was stable across the stages (~12 min for intrinsic and ~5.5 min for extrinsic pathways). In the intrinsic pathway, MCF significantly reduced from the baseline (1.18±0.02) to 1.12±0.02 at the artery stage and slightly increased to 1.14±0.02 at the final stage; meanwhile, MCF in the extrinsic pathway decreased from 1.17±0.03 at the baseline to 1.13±0.02 at the artery stage and remained at the same value at the final stage (same trends observed in TEG).
For PPP samples triggered via intrinsic pathway, MCF reduced from 1.13±0.02 (baseline) to 1.08±0.01 (artery) and increased to 1.12± 0.01 (final). In extrinsic pathway, the MCF values were 1.15±0.03 (baseline), 1.1±0.02 (artery), and 1.14±0.01 (final).
We see that blood samples at the artery stage are less stiff than the samples at the baseline and final stages due to reduction in fibrinogen concentration, as seen for most liver transplant patients.
Conclusions Using a single drop of blood per test, the i-QATTTM technique provides comprehensive information on blood coagulation status in liver transplant patients undergoing blood transfusion. This technique can be potentially adopted for transfusion guidance in perioperative settings.
Disclosures
Kasireddy:Levisonics Inc.: Current Employment. Khismatullin:Levisonics Inc.: Current equity holder in publicly-traded company, Patents & Royalties: PCT/US14/55559 (pending); Levisonics Inc.: Patents & Royalties: PCT/US2018/014879 (issued) ; Levisonics Inc.: Patents & Royalties: PCT/US21/15336 (pending)...
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal